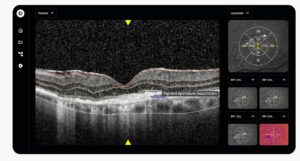
Ikerian (parent company of RetinAI U.S. Inc.) receives EU-MDR Certificate for four devices, its Ophthalmology Data Platform and AI models
Bern & Boston, US, June 24th, 2024 – Ikerian AG (“Ikerian”) a leader in developing software solutions for medical image and data management and artificial intelligence (AI) in healthcare, today announced the registration as Class IIa medical devices under the European Union Medical Device Regulation (Regulation (EU) 2017/745) (EU-MDR) of 4 devices.