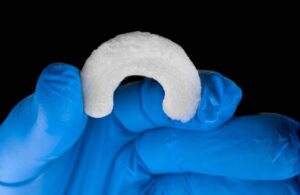
FDA clears Regenity Biosciences’ regenerative meniscus implant
Regenity Biosciences announced that it received FDA 510(k) clearance for its RejuvaKnee implant for soft tissue injuries of the meniscus.

Regenity Biosciences announced that it received FDA 510(k) clearance for its RejuvaKnee implant for soft tissue injuries of the meniscus.
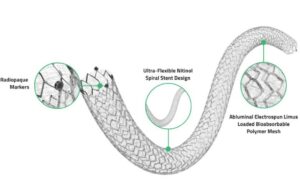
Medinol today announced the successful first-in-human implantation of its ChampioNIR drug-eluting peripheral stent.
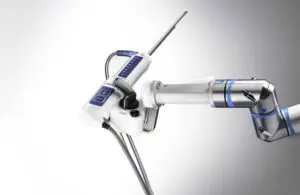
Procept BioRobotics (Nasdaq:PRCT) announced today that the FDA approved an investigational device exemption (IDE) trial for its Aquablation therapy.

CHICAGO, Oct. 3, 2024 /PRNewswire/ — Amphix Bio, a company developing a new class of regenerative medicine therapies, announced today it has received a Breakthrough Device designation from the U.S. Food and Drug Administration (FDA) for a drug-device combination product for bone regeneration. The designation covers the use of the therapeutic device to treat degenerative disc disease with transforaminal lumbar interbody fusion (TLIF) procedures.
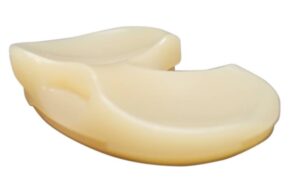
Corin announced that it received FDA 510(k) clearance for its Unity Knee medial constrained (MC) tibial insert.
Pi-Cardia designed the Shortcut device to mitigate the risk of coronary artery obstruction by splitting aortic valve leaflets before valve placement.

GE HealthCare’s MIM Software today announced it received FDA 510(k) clearance for Monte Carlo dosimetry.
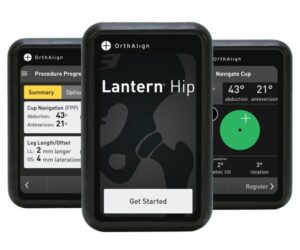
IRVINE, Calif., Oct. 3, 2024 /PRNewswire/ — OrthAlign, Inc., a privately held medical device company, announces FDA 510(k) clearance of their Lantern Hip handheld technology for direct anterior total hip arthroplasty with the patient in the supine position. Lantern Hip is the latest addition to the Lantern platform, joining existing applications for total knee, revision knee, and partial knee arthroplasty.

A 2025 commercial launch is planned to expand access to this platform technology.
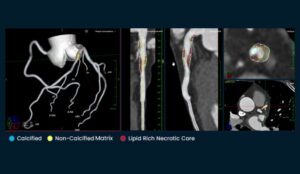
The company says this is the first and only software validated on ground-truth histology, the gold standard for plaque characterisation according to the company.