New Soldier® High Flow Microcatheter Granted FDA Clearance
FDA Clears Soldier Microcatheter for Localized Drug Delivery in Vascular Interventions

FDA Clears Soldier Microcatheter for Localized Drug Delivery in Vascular Interventions
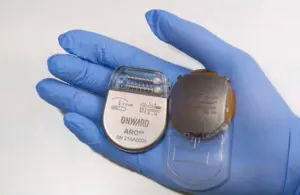
Onward Medical announced today that the FDA granted breakthrough device designation for its ARC-BCI system that uses brain-computer interface (BCI) technology.

TYLER, Texas–(BUSINESS WIRE)– AIOMEGA, a Texas biomedical company, announced that AIO BREATHE, their medical device that treats Obstructive Sleep Apnea, has been cleared by the Food and Drug Administration.

Nevro1™ Proven to Immediately Transfix Sacroiliac (SI) Joint to Allow for Long-term SI Joint Fusion

Sonus Microsystems has unveiled its latest ultrasound technology, delivering remote diagnostic imaging, without the need to go to a clinic or hospital

TOKYO, Feb. 27, 2024 /PRNewswire/ — PENTAX Medical, a division of HOYA Group, have obtained CE marks for new models of PENTAX Medical i20c Video Endoscope Series – PENTAX Medical Video Colonoscope EC34-i20c, PENTAX Medical Video Upper GI Scope EG27-i20c and R/L Knob Adaptor OE-B17.
Virtual Incision announced that the FDA granted marketing authorization to its MIRA miniaturized surgical robotic system.

The clearance poises the orthopedics company to be first to market with robotic-assisted shoulder replacement surgery.

Allowance for U.S. Patent Application Serial Number 18/107,536 for Tenon’s Catamaran® SI Joint implant for stabilizing a dysfunctional sacroiliac (SI) joint.

For use in children weighing 10 kilograms or more with acute kidney injury (AKI) due to sepsis or a septic condition requiring kidney replacement therapy (KRT).