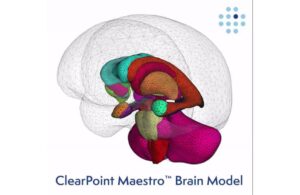
New ClearPoint Neuro software wins FDA clearance, used in first cases
ClearPoint Neuro (Nasdaq:CLPT) announced that the FDA cleared its ClearPoint 2.2 software with integrated Maestro Brain Modeling.

ClearPoint Neuro (Nasdaq:CLPT) announced that the FDA cleared its ClearPoint 2.2 software with integrated Maestro Brain Modeling.
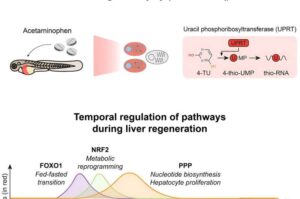
Researchers at Peter Mac have made a key discovery in liver regeneration that may have important implications for liver cancer. Joint research by Associate Professor Andrew Cox and Professor Mark Dawson, published Feb. 15 in Developmental Cell, has identified how the liver is triggered to regrow when damaged.

Virginia Tech researchers at the Fralin Biomedical Research Institute at VTC report that applying low-intensity focused ultrasound to an area deep within the brain may point to new ways to help people cope with chronic pain.
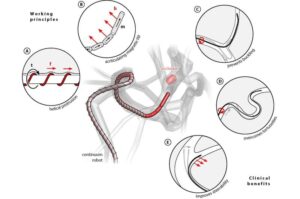
A team of roboticists at Multi-Scale Robotics Lab, ETH Zurich, working with several hospitals in Switzerland, has developed a magnetically operated robot that could potentially be used to treat people after a stroke. Their paper is published in the journal Science Robotics.

Merit Medical Systems (Nasdaq:MMSI) announced today that it received FDA 510(k) clearance for the Scout MD surgical guidance system
MIT spinout Elicio developed a vaccine based on a lymph node-targeting approach first developed at the Koch Institute. Phase 1 solid tumor clinical trial results are promising so far.

The company’s OTC device, which costs nearly $300, will compete with consumer pulse oximeters that are widely available but lack FDA clearance.

The FDA created special controls that provide a path to market for other developers of over-the-counter devices.
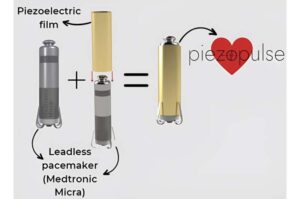
Heartbeats could one day help power something beyond hearts. A pacemaker is a device that helps regulate abnormal heart rates using electrical pulses. Leadless pacemakers, which are placed in the heart and don’t have wires like conventional transvenous pacemakers, are becoming more popular. However, their batteries only last five to 12 years, and it’s difficult to retrieve the device when the battery is drained.

SAN FRANCISCO, Feb. 14, 2024 (GLOBE NEWSWIRE) — ZKR Orthopedics, Inc., a clinical stage medical device company, announced that it has received Investigational Device Exemption (IDE) approval from the United States Food and Drug Administration (FDA) for a multicenter prospective clinical trial. The approved trial will evaluate the Company’s LIFT implant to treat later stage patellofemoral cartilage degeneration in adult patients.