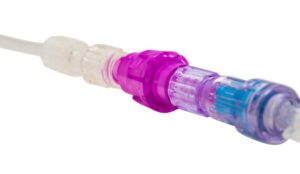
FDA grants expanded indication for Linear Health Sciences’ Orchid Safety Release Valve
The IV dislodgement-prevention device is now approved for use with blood or blood product transfusions.

The IV dislodgement-prevention device is now approved for use with blood or blood product transfusions.

The ZEISS MICOR 700 uses the ZEISS NULEX (non-ultrasonic lens extraction) procedure to help surgeons broaden their intraocular working space.

Axonics (Nasdaq:AXNX) announced today that it received regulatory approval in Australia for its R20 rechargeable sacral neuromodulation (SNM) system.
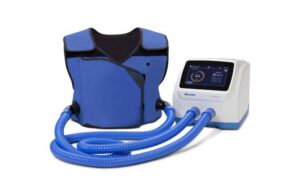
Baxter (NYSE: BAX)+
today unveiled its next-generation airway clearance system, The Vest advanced pulmonary experience (APX) system.

GAINESVILLE, Fla., Sept. 25, 2024 /PRNewswire/ — Exactech, a global medical technology leader, announced today the successful completion of the first ankle replacement surgeries using its new Vantage® Ankle 3D and 3D+ tibial implants.

Nevro (NYSE:NVRO) announced today that it received FDA approval for an AI-powered, personalized pain management platform for spinal cord stimulation.

Neurable announced today that it launched the MW75 Neuro smart headphones integrated with its brain-computer interface (BCI) technology.
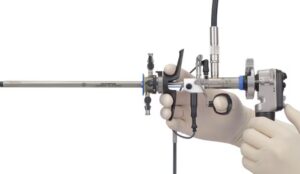
The new device offers unified visualisation with true 4K Imaging and 4K narrow band imaging as well as blue light observation.
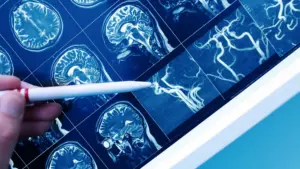
Nico sells devices for navigating through the brain, visualizing the organ, removing tumors and clots and collecting tissue.
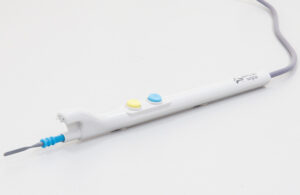
Alesi Surgical has received FDA 510(k) clearance of its Ultravision2 IonPencil for cutting and coagulating soft tissue in open surgery while managing surgical smoke.