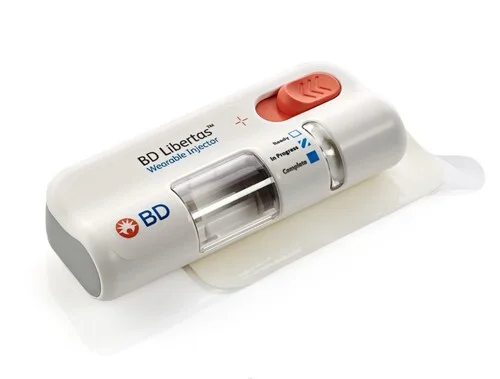
BD’s announcement of the first pharma-sponsored clinical trial using its BD Libertas™ Wearable Injector marks a significant advancement in patient-centered biologic therapy. As more complex biologic drugs enter the market—often requiring hospital-based infusion—the demand for self-administered, subcutaneous delivery systems is growing rapidly. The BD Libertas™ system answers this need with a prefilled, ready-to-use design that eliminates cumbersome setup steps and empowers patients to manage treatment outside of clinical settings. This trial represents a major step forward in validating wearable injectors as a safe, effective alternative to traditional administration methods, reducing the burden on healthcare infrastructure while enhancing patient autonomy.
Designed to support delivery of high-viscosity drugs and large-volume doses (up to 10 mL), the BD Libertas™ Wearable Injector offers flexibility for a broad range of therapies—from oncology and immunology to chronic inflammatory conditions. Its mechanical, user-friendly design simplifies drug administration with a “peel, stick, and click” mechanism, addressing a common barrier to at-home treatment adoption. In BD-led clinical studies, the device demonstrated excellent usability and patient acceptance, with all participants expressing confidence in using the injector if prescribed. For pharmaceutical developers, this system offers a customizable platform that can accelerate time-to-market for combination products and expand access to biologics.