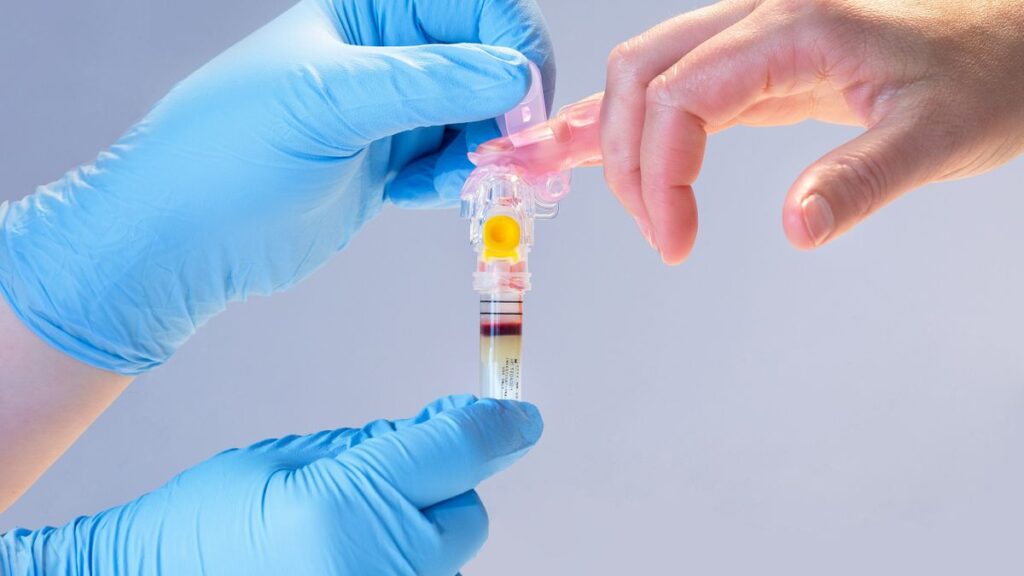
Becton Dickinson has received 510(k) clearance for a device that collects blood from a fingerstick instead of from the vein. The MiniDraw Capillary Blood Collection System container is slightly larger than its predicate device, the BD Microtainer MAP Microtube, but is cleared for sample collection in “ancillary healthcare facilities,” positioning BD and its partner Babson Diagnostics to support blood collection from community sites such as pharmacies. When Babson rolls out its BetterWay blood testing service next year, the device could support the collection of samples for lipid panel, selected chemistry tests, and hemoglobin and hematocrit analyses.