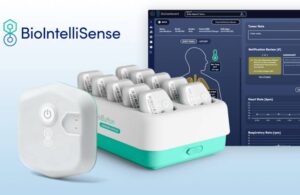
The BioButton device expands the company’s portfolio of continuous patient monitoring solutions with a rechargeable, reusable inpatient solution. It automates vital sign collection across medical surgical units, specialty care areas and emergency departments. The company says it delivers hospital-level care in the home.
Denver-based BioIntelliSense’s BioDashboard clinical intelligence system enables scalable, automated trending notifications. It personalizes the notifications for each patient, helping one clinician monitor hundreds of BioButton users simultaneously. BioDashboard’s configurable dashboard view enables proactive clinical decision-making as well.