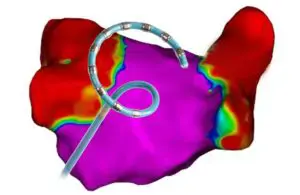
Varipulse treats symptomatic drug-refractory recurrent paroxysmal AFib using pulsed field ablation (PFA). The platform features the Varipulse catheter, a variable-loop multielectrode catheter, the TruPulse generator and the Carto 3 3D cardiac mapping system.
Biosense Webster says this marks the first and only PFA system approval in Japan.
According to a news release, Varipulse is the first and only Carto-integrated PFA system. It enables an intuitive and reproducible workflow with real-time visualization and feedback mechanisms.
Biosense Webster’s inspIRE trial’s early clinical results demonstrated a one-year clinical success of 78.9% with Varipulse. The trial defined success as freedom from documented symptomatic atrial arrhythmia recurence. inspIRE also demonstrated a notable safety profile with zero primary adverse events.