MedTech News
.................... by Andrew Celentano

Potential new therapeutic target for asthma discovered
A new way to treat asthma symptoms and even repair previously irreversible lung damage could be on the horizon following the discovery of a potential new therapeutic target by scientists at the Universities of Aberdeen and Manchester.

Brain shape changes could offer early warning signs of dementia
A new study led by University of California, Irvine’s Center for the Neurobiology of Learning and Memory researchers found that aging changes the brain’s overall shape in measurable ways. Instead of focusing only on the size of specific regions, the team used a new analytic method to see how the brain’s form shifts and distorts over time.
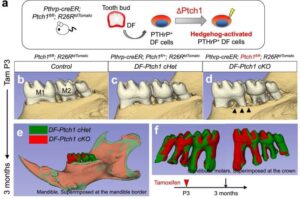
Stem cell studies could pave way for regenerating lost teeth
Two distinct stem cell lineages that drive tooth root and alveolar bone formation have been identified by researchers from Science Tokyo. Using genetically modified mice and lineage-tracing techniques, the team has shed light on the cell signaling mechanisms guiding differentiation in stem cells in the developing teeth, offering key insights for future regenerative dental therapies.
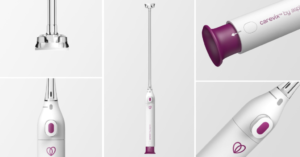
Carevix device launches in Southern California & New York
Aspivix, a FemTech company specialising in the modernisation of gynaecological care, has announced the official launch of its breakthrough device, carevix, now available to healthcare professionals in Southern California and New York State.
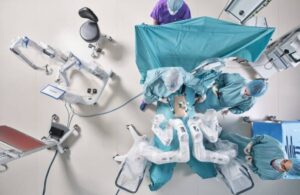
FDA clears Distalmotion Dexter surgical robot for hysterectomy
Distalmotion announced today that it received FDA 510(k) clearance for the use of its Dexter robotic surgery system in hysterectomy.
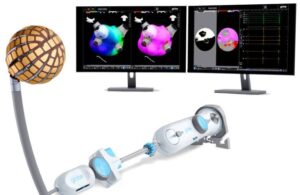
Kardium reports first commercial cases with Globe PFA
Kardium announced today that it completed the first commercial procedures using its Globe pulsed field ablation (PFA) system.
Medtronic wins CE mark for left atrial appendage exclusion system
Medtronic (NYSE: MDT)+
announced today that it received CE mark approval for its Penditure LAA exclusion system.
Aidoc Receives FDA Breakthrough Device Designation for First-of-Kind AI Solution Spanning Numerous Acute Conditions in CT
NEW YORK, Sept. 30, 2025 /PRNewswire/ — Aidoc, the global leader in clinical AI, today announced the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation for the company’s novel multi-triage solution that flags a wide array of life-threatening, time-sensitive medical conditions, all within a single workflow. Built on CARE™, the first clinical-grade foundation model in healthcare with FDA cleared solutions, and deployed through Aidoc’s aiOS™ platform, the solution is designed to help care teams attend to high–risk cases faster and more consistently across the health system.
