MedTech News
.................... by Andrew Celentano
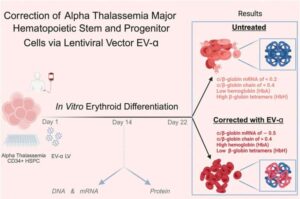
One-time gene therapy could end lifelong transfusions for rare blood disease
Thanks to in-utero blood transfusion technology, what was once a fatal diagnosis in the womb can now result in live births. However, this medical advancement created a new challenge: a growing population of children born with that diagnosis—the severe, inherited blood disorder alpha thalassemia—which requires lifelong specialized care.

AI model offers accurate and explainable insights to support autism assessment
Scientists have developed and tested a deep-learning model that could support clinicians by providing accurate results and clear, explainable insights—including a model-estimated probability score for autism.

Shape-shifting material could transform future of implantable and ingestible medical devices
Researchers led by Rice University’s Yong Lin Kong have developed a soft but strong metamaterial that can be controlled remotely to rapidly transform its size and shape.
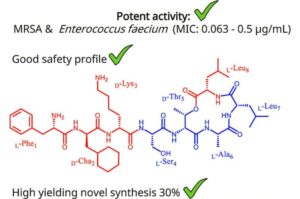
Scientists discover powerful new antibiotic class to tackle deadly superbugs
Scientists at the University of Liverpool, working with international collaborators, have discovered Novltex, a groundbreaking new class of antibiotics with potent activity against some of the world’s most dangerous multidrug-resistant (MDR) bacteria.
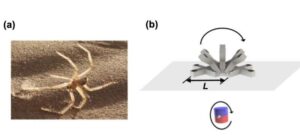
Spider-inspired magnetic soft robots could perform minimally invasive gastrointestinal tract procedures
A team of researchers at the University of Macau in China recently developed new soft magnetic robots that can climb inverted surfaces and move in complex environments, which could allow them to deliver drugs to specific locations in the GI tract.
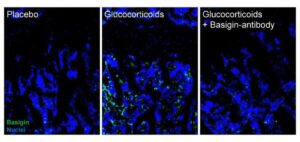
Researchers uncover a key protein and a promising strategy to prevent bone damage from steroids
A new study from UC Davis Health researchers reveals a promising new way to treat various bone loss conditions, including age-related osteoporosis.
FDA clears Amber Implants’ vertebral augmentation system
The company is working to obtain CE marking of the system in the European Union.
OrganOx gets FDA green light for liver perfusion during air transport
OrganOx announced today that it received FDA approval for the use of its normothermic machine perfusion (NMP) device during air transport.
