MedTech News
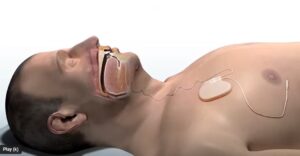
LivaNova submits nerve stim for sleep apnea to FDA, raises 2025 guidance
LivaNova (Nasdaq: LIVN)+
today announced 12-month data from its OSPREY study supporting an FDA submission of the aura6000 system.
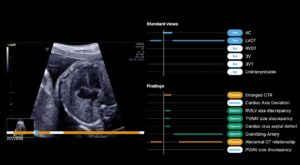
FDA clears more BrightHeart AI capabilities in fetal heart ultrasound
BrightHeart earned its second FDA 510(k) clearance for updates to its BrightHeart platform, an AI-powered digital screening tool for congenital heart defects (CHDs).

Bausch + Lomb wins CE mark for full range of vision intraocular lens
Bausch + Lomb announced today that it received CE mark approval for its LuxLife full range of vision intraocular lens (IOL).
Function Health Acquires Ezra, Introduces Full Body MRI with an FDA-cleared AI that cuts scan time from 60 minutes to 22 minutes
This historic acquisition reduces barriers that prevent people from taking control of their lifelong health. Full Body MRI scan now takes just 22 minutes instead of 60 minutes, costs $499 instead of over $1,500
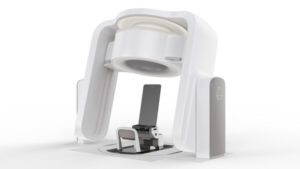
Leo Cancer Care Particle Therapy Solution Marie is now 510(k) Pending
MADISON, Wis., May 6, 2025 /PRNewswire/ — The Food and Drug Administration Agency (FDA) has updated the regulatory status of Marie®, Leo Cancer Care’s upright particle therapy solution, to pending. Marie combines Leo Cancer Care’s upright patient positioning system with their upright fan beam CT scanner and can be utilized with any fixed particle beam.
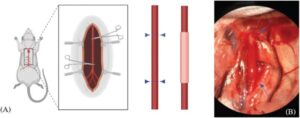
Bioprinted aortas successfully implanted in rats, offering new hope for vascular repair
Yale researchers have built a 3D-bioprinted synthetic aorta that they have successfully implanted into rats. This technology could advance the treatment of cardiovascular diseases such as coronary artery disease or peripheral arterial disease by allowing scientists to engineer and replace blood vessels in humans.
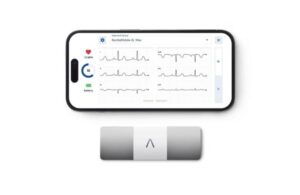
AliveCor launches latest AI-powered personalized ECG
AliveCor announced today that it launched its most advanced personal ECG system, the AI-powered KardiaMobile 6L Max.
Shingles vaccine lowers the risk of heart disease for up to eight years, study finds
People who are given a vaccine for shingles have a 23% lower risk of cardiovascular events, including stroke, heart failure, and coronary heart disease, according to a study of more than a million people published in the European Heart Journal.
