MedTech News
.................... by Andrew Celentano

Schizophrenia and osteoporosis share 195 genetic loci, highlighting unexpected biological bridges between brain and bone
A comprehensive genetic investigation led by Dr. Feng Liu at Tianjin Medical University General Hospital has uncovered striking molecular connections between schizophrenia and bone health, identifying 195 shared genetic loci that may explain why psychiatric patients face elevated fracture risks.

Robot-assisted therapy beneficial for children with autism
Social robots are efficacious and effective for children with autism, according to a study published online Dec. 24 in Science Robotics.
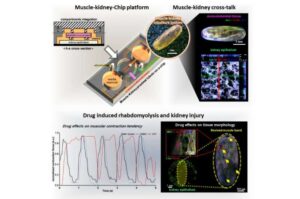
Organ-on-a-chip simulates drug-triggered muscle and kidney injury
KAIST researchers have developed a new device that can precisely reproduce how muscle and kidney damage influence each other simultaneously within the human body.
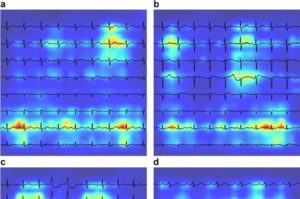
AI-powered ECG analysis shows promise for early COPD detection
A study published in the journal eBioMedicine assesses the effectiveness of electrocardiograms (ECGs) analyzed via deep learning as a tool for early COPD detection.
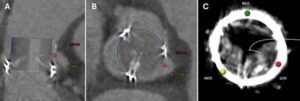
First minimally invasive coronary artery bypass achieved
For high-risk patients, the method could offer a safer alternative to open-heart surgery.

New AI model predicts disease risk while you sleep
A poor night’s sleep portends a bleary-eyed next day, but it could also hint at diseases that will strike years down the road. A new artificial intelligence model developed by Stanford Medicine researchers and their colleagues can use physiological recordings from one night’s sleep to predict a person’s risk of developing more than 100 health conditions.
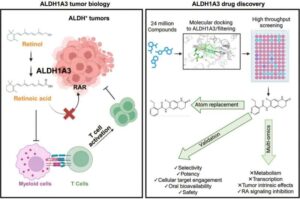
Immune sabotage: How a Vitamin A byproduct compromises the body’s normal anti-cancer response
Scientists at the Princeton University Branch of the Ludwig Institute for Cancer Research have identified novel mechanisms by which a metabolic derivative of vitamin A—all-trans retinoic acid—compromises both the body’s normal anti-cancer immune response and, in a different context, the efficacy of a promising type of cancer vaccine.
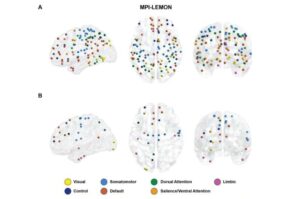
Mathematics uncovers shifting brain connectivity in autism and aging
Researchers from the Max Planck Institute for Mathematics in the Sciences Leipzig, Germany, the Institute of Mathematical Sciences in Chennai, India, and colleagues demonstrate how mathematical techniques from topological data analysis (TDA) can provide a new, multiscale perspective on brain connectivity.
