MedTech News
.................... by Andrew Celentano
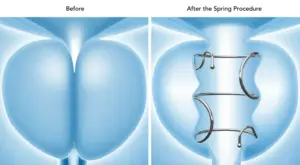
FDA Approves Zenflow Spring Implant and Delivery System for BPH
The Spring is the only FDA-approved FIT in a range of lengths and diameters, allowing for patient personalisation

European researchers developed energy-efficient machine vision inspired by human eyesight and the brain
A European research project led by VTT has developed machine vision mimicking the cooperation of the eye and nervous system, implemented as edge-computing circuits.

Vesalio Receives FDA 510(k) Clearance of enVast, the First Stent-Based Coronary Thrombectomy Technology
PLANO, Texas, Dec. 11, 2025 /PRNewswire/ — Vesalio, a leader in thrombectomy solutions, today announced FDA 510(k) clearance and the upcoming U.S. commercial launch of enVast™, the first and only clot retriever specifically cleared for mechanical thrombectomy in the cardiac circulation. enVast introduces a proven, innovative approach to clot capture and removal, redefining coronary thrombectomy for patients with large thrombus burden (LTB).
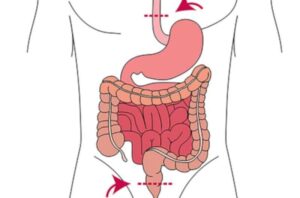
New sensor technology can detect life-threatening complications after intestinal surgery at an earlier stage
An interdisciplinary research team from Dresden University of Technology (TUD), Rostock University Medical Center (UMR) and Dresden University Hospital has developed an innovative, implantable and fully absorbable sensor film.
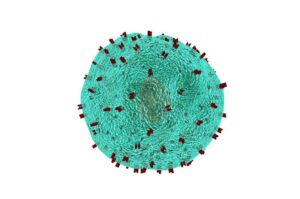
CAR-T therapy yields long-term survival for patients with lymphoma
A study published in the Journal of Clinical Oncology confirms that one of the first Food and Drug Administration-approved CAR-T cell therapies offers long-term survival and potential cures for adult patients with relapsed or refractory large B-cell lymphoma, even years after treatment.
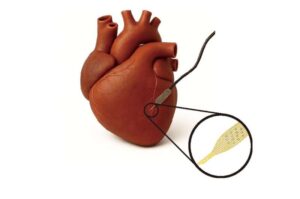
Soft ‘cyborg’ cardiac patches could improve stem cell heart repair
Heart muscle cells grown from patient stem cells—known as human induced pluripotent stem cell–derived cardiomyocytes, or hiPSC-CMs—are a promising way to repair hearts damaged by heart attacks and heart failure.

Neurovalens wins FDA nod for weight management neurostim device
Neurovalens announced that it received FDA de novo approval for Modius Lean, its non-invasive neurotechnology for weight management.

FDA clears Flow Neuroscience’s at-home depression treatment in US first
Flow Neuroscience’s at-home depression treatment device has become the first of its kind to be approved by the FDA.
