MedTech News
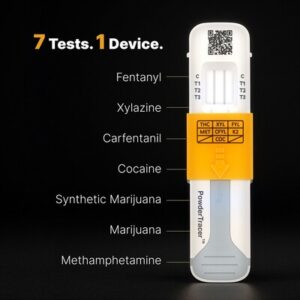
New Forensic Tool Detects 7 Drugs in Under 5 Minutes
SAN DIEGO, April 23, 2025 /PRNewswire/ — Biolabs International LLC proudly introduces PowderTracer™, a groundbreaking, rapid surface test, and its companion app, designed to swiftly detect seven dangerous drugs. This innovative tool is poised to revolutionize forensic drug detection in the field, providing accurate results in under five minutes. PowderTracer™ addresses the urgent need for rapid, reliable drug identification, particularly in the face of the escalating opioid crisis and the increasingly sophisticated methods used by drug traffickers.
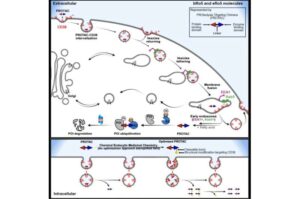
Drug uptake discovery could allow IV medications to be taken orally
A team of scientists has uncovered the mechanism of cellular uptake for large and polar drugs and devised a novel strategy to optimize the capacity of drug-delivery into these cells. The team was led by Hong-yu Li, Ph.D., professor of medicinal chemistry and chemical biology with the Department of Pharmacology and the Barshop Institute at the University of Texas Health Science Center at San Antonio (UT Health San Antonio), together with teams from Duke University (Duke) and the University of Arkansas for Medical Sciences (UAMS)
Orchestra BioMed wins FDA breakthrough nod for hypertension treatment
Orchestra BioMed (Nasdaq:OBIO) announced today that it received FDA breakthrough device designation for its atrioventricular interval modulation (AVIM) therapy.

Novocure wins CE mark for its lung cancer treatment wearable
Novocure (Baar, Switzerland) announced today that it has won a CE mark for its Optune Lua wearable device for treating metastatic non-small cell lung cancer.
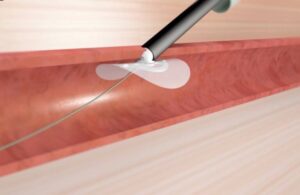
Vivasure wins CE mark for PerQseal Elite vascular closure system
Vivasure Medical announced today that it received CE mark approval for its PerQseal Elite vascular closure system.
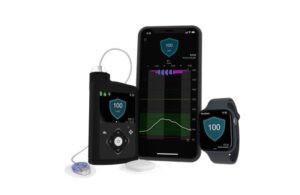
Medtronic wins FDA nod for new Simplera Sync CGM with MiniMed 780G
Medtronic (NYSE:MDT) announced that the FDA approved its Simplera Sync continuous glucose monitor for use with the MiniMed 780G.
ARUP Launches pTau 217 Blood Test To Detect Alzheimer’s Disease Pathology
SALT LAKE CITY, April 21, 2025 /PRNewswire/ — ARUP Laboratories now provides a blood test for phosphorylated tau 217 (pTau 217) to assist in identifying whether cognitive decline symptoms in patients ages 60 years and older are related to Alzheimer’s disease (AD) pathology. As this biomarker can be detected in blood, this test is a minimally invasive and broadly accessible diagnostic tool that may facilitate earlier detection of AD.

Soft brainstem implant delivers high-resolution hearing
Now, a team at EPFL’s Laboratory for Soft Bioelectronic Interfaces has developed a soft, thin-film ABI. The device uses micrometer-scale platinum electrodes embedded in silicone, forming a pliable array just a fraction of a millimeter thick.
