MedTech News
.................... by Andrew Celentano
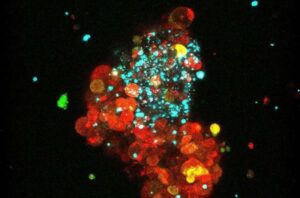
New approach makes common breast cancer type responsive to immunotherapy
A study led by researchers at the Hospital del Mar Research Institute advances one of the most significant milestones in breast cancer treatment, making immunotherapy effective against the most common tumor type, estrogen receptor-positive or luminal breast cancer.
Genetic testing reveals often-overlooked fungal infections in California clinics
A new study reveals that a rarely-diagnosed and frequently drug-resistant species, Aspergillus tubingensis, may be one of the most common causes of fungal infections in Southern California.
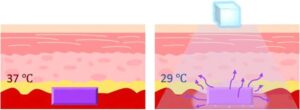
Innovative drug delivery mechanism triggered by cooling could provide targeted pain relief
Leon Bellan, associate professor of mechanical and biomedical engineering at Vanderbilt, and his team have developed a novel cooling-triggered device that could allow patients to safely and conveniently receive drugs for pain relief.

Miniature microscope captures real-time voltage signals in awake animals
Researchers have built a tiny, lightweight microscope that captures neuron activity with unprecedented speed that can be used in freely moving animals. The new tool could give scientists a more complete view of how brain cells process information during natural behavior.

Dance effective in fighting against cognitive decline in Parkinson’s, study finds
A new study led by researchers at York University shows that dance can be beneficial in halting the cognitive decline associated with Parkinson’s disease and, for some participants, they even showed signs of improvement.

Medtronic earns expanded FDA nod for deep brain stim
Medtronic (NYSE: MDT)+
announced that the FDA approved expanded labeling for its deep brain stimulation (DBS) offering.

Cardiawave wins CE mark for non-invasive aortic stenosis treatment
Cardiawave announced that it received CE mark approval for Valvosoft, its non-invasive therapeutic alternative for treating aortic stenosis (AS).

Axolotls Can Regrow a Key Organ From Scratch, Allowing Immune Cells to Fight Infections
Learn how axolotls are able to fully regenerate their thymus, a small organ that trains immune cells to fight infections.
