MedTech News
.................... by Andrew Celentano

Bacterial enzyme may cause fatal heart conditions with pneumonia infections
Pneumonia is a disease that burdens the health care system with more than 1.2 million emergency room visits each year and more than 41,000 adult deaths in the United States. Worldwide, more than one million children under the age of five die of the disease annually.
New organ-on-a-chip platform allows the testing of cancer vaccine efficacy in aging populations
Dr. Vadim Jucaud’s lab at the Terasaki Institute has introduced a new organ-on-a-chip platform that recapitulates age-dependent immune responses, offering a more accurate testing bed for evaluating cancer vaccine performance in older adults, the population most affected by cancer and often overlooked in traditional preclinical testing.

Antibody halts triple-negative breast cancer in preclinical models
Triple-negative breast cancer (TNBC) is one of the most aggressive and treatment-resistant forms of breast cancer.

Insulet wins FDA clearance for Omnipod 5 algorithm enhancements
Insulet (Nasdaq: PODD)+
announced today that it received FDA 510(k) clearance for new enhancements to its Omnipod 5 system.
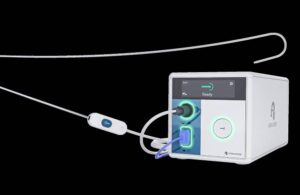
Atraverse wins FDA clearance for left heart access device
Atraverse Medical announced today that it received FDA clearance for its fully integrated Hotwire transseptal access system.

Boston Scientific gets European nod for Farapoint catheter
Boston Scientific (NYSE: BSX)+
announced recently that it received CE mark for its Farapoint pulsed field ablation (PFA) catheter.
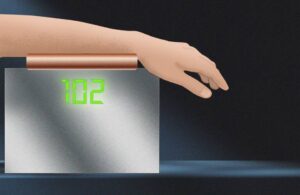
Researchers say they can use imaging to measure blood glucose for people with diabetes
Researchers at MIT developed a non-invasive method for measuring blood glucose levels, potentially offering an alternative to fingersticks.
FDA grants IDE approval for Renerva’s trial of nerve capping device
The trial will enroll patients who are undergoing nerve management procedures for severe neuropathic pain.
