MedTech News
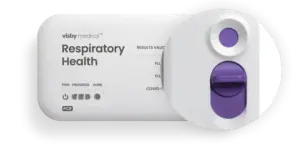
Visby Medical™ Receives FDA Clearance and CLIA Waiver for Point-of-Care Respiratory Health Test
SAN JOSE, Calif., Feb. 26, 2025 /PRNewswire/ — Visby Medical™ announced today that it has received 510(k) clearance and was granted a CLIA waiver from the U.S. Food and Drug Administration (FDA) for its point-of-care respiratory health test. The Visby Medical Respiratory Health Test is a rapid polymerase chain reaction (PCR) test that detects and differentiates between upper respiratory infections caused by influenza (Flu) A & B, and SARS-CoV-2 (COVID-19). This multiplexed molecular device is the first handheld test to receive this designation after being granted Emergency Use Authorization (EUA) in December 2022.

Sway Medical, Inc. Announces FDA 510(k) Clearance of its Comprehensive Concussion Management System
TULSA, Okla., Feb. 26, 2025 /PRNewswire/ — Sway Medical, Inc., the company that created the Mobile Concussion Management category, is proud to announce that it has received FDA 510(k) clearance as a Computerized Cognitive Assessment Aid for Concussion under Section 882.1471. This clearance expands on Sway Medical’s previous FDA clearance for balance testing in head injuries, officially recognizing Sway as the first fully integrated tool that combines both cognitive and balance testing into one product for concussion management.

New blood test identifies organ aging, links to 30 future diseases
Our organs age at different rates, and a blood test determining how much they’ve each aged could predict the risk of conditions like lung cancer and heart disease decades later, finds a new study led by University College London (UCL) researchers.
Blood test could lead to better diagnosis and management of ALS
ALS, or amyotrophic lateral sclerosis, can sometimes be difficult to diagnose or to predict how quickly the disease is likely to progress. A new study helps determine which blood tests are best at identifying and monitoring ALS. The study is published in the online issue of Neurology.
Glioblastoma treatment strategy reprograms cancer cells, halting tumor growth
UCLA scientists have identified a potential new strategy for treating glioblastoma, the deadliest form of brain cancer, by reprogramming aggressive cancer cells into harmless ones.

A protein from tiny tardigrades may help cancer patients tolerate radiation therapy
When scientists stimulated cells to produce a protein that helps “water bears” survive extreme environments, the tissue showed much less DNA damage after radiation treatment.

OrthoNovis, Inc. Receives FDA 510(k) Clearance to Market Innovative Wrist Fracture System
PALM COAST, Fla., Feb. 25, 2025 /PRNewswire/ — OrthoNovis, Inc is proud to announce that it has received U.S. Food and Drug Administration (FDA) clearance to market its innovative BPS Wrist Fracture System. www.orthonovis.com
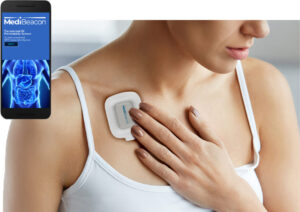
MediBeacon® Transdermal GFR System receives device approval in China; Peer-reviewed articles on MediBeacon technology published
ST. LOUIS, Feb. 25, 2025 /PRNewswire/ — MediBeacon Inc. today announced the National Medical Products Administration (NMPA) in China has approved the MediBeacon® TGFR Monitor and TGFR Sensor for the assessment of kidney function in patients with normal or impaired renal function. Lumitrace® (relmapirazin) injection, categorized as a drug in China, is under review and is targeted for approval in late 2025. The transdermal GFR technology includes Lumitrace (relmapirazin) injection, a non-radioactive, non-iodinated fluorescent GFR tracer agent, which together with the TGFR Monitor and TGFR Sensor allow assessment of kidney function by measuring the clearance rate of the fluorescent agent as it leaves the body.
