MedTech News
.................... by Andrew Celentano

Overlooked hormone may be deadly driver of postmenopausal breast cancer in women with obesity
A new analysis of research into the most common type of breast cancer has zeroed in on an overlooked hormone that may be responsible for the increased risk of breast cancer death in postmenopausal women with obesity.
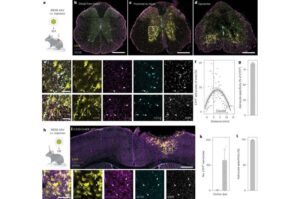
How the nervous system activates repair after spinal cord injury
After a spinal cord injury, cells in the brain and spinal cord change to cope with stress and repair tissue. A new study from Karolinska Institutet, published in Nature Neuroscience, shows that this response is controlled by specific DNA sequences. This knowledge could help develop more targeted treatments.

In-home sensor technology offers smarter care for ALS patients
Bill Janes is on a mission to improve life for people with amyotrophic lateral sclerosis (ALS). As a licensed occupational therapist and researcher at the University of Missouri, he’s seen firsthand how the disease can steal a person’s strength, speech and independence.
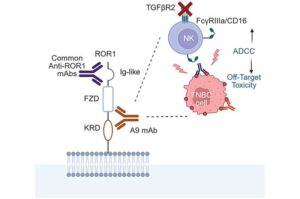
Antibody designed to guide immune cells against hard-to-treat cancer types
A cancer-targeting antibody that helps the body’s immune cells spot and destroy hard-to-treat tumors such as triple-negative breast cancer has been developed by researchers.

Blocking Claudin-4 protein may help immune system fight aggressive ovarian cancer
Scientists at The University of Texas at El Paso have found a promising new target in the fight against high-grade serous carcinoma, an aggressive form of ovarian cancer. Less than 50% of women survive five years after diagnosis, according to the team.
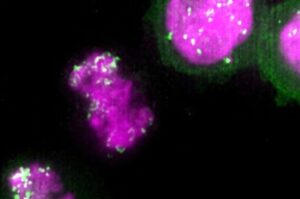
Cancer-promoting DNA circles hitchhike on chromosomes to spread to daughter cells
Small, cancer-associated DNA circles “hitchhike” on chromosomes during cell division to spread efficiently to daughter cells by co-opting a process used to maintain cellular identity through generations, Stanford Medicine-led research has found.
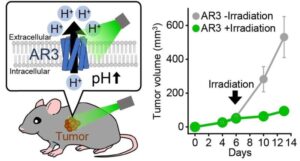
Light-activated protein triggers cancer cell death by raising alkalinity
One of the hallmarks of cancer cells is their ability to evade apoptosis, or programmed cell death, through changes in protein expression.
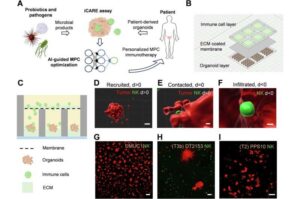
Cancer-fighting bacterial product ‘cocktails’ may offer personalized treatment
Bacteria may be the next frontier in cancer treatment, according to a team led by researchers at Penn State that devised a new approach of creating bacteria-derived mixtures—or cocktails—to help fight bladder cancer.
