The FDA also accepted the system into its Total Product Life Cycle (TPLC) Advisory Program (TAP) Pilot. TAP provides early and frequent strategic engagement from the FDA, patients, providers and payers. It facilitates rapid development and widespread access to medical devices.
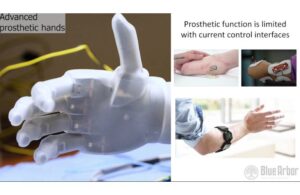
Grass Lake, Michigan-based Blue Arbor designed Restore to integrate into the peripheral nervous system with available robotic prosthetics. It aims to restore naturalistic hand and arm function in patients with upper limb loss. The platform could enable patients to move upper limb prosthetic devices with improved dexterity, speed and reliability.