Lung cancer remains a leading cause of cancer-related death in Brazil, responsible for approximately 29,000 deaths annually, according to data from the Brazilian National Cancer Institute (INCA). Late diagnosis continues to be a challenge, with the majority of patients diagnosed at an advanced stage, where curative treatment options are limited.
“Securing regulatory approval in Brazil is a key milestone in our expansion across Latin America,” said Benny Krauz, Vice President of Regulatory and Quality at Body Vision Medical. “We’re proud to partner with Supri to make LungVision available to Brazilian physicians and patients, and to help raise the standard of care for early lung cancer diagnosis.”
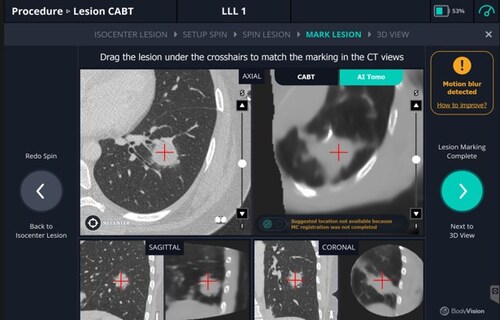
LungVision transforms any standard C-arm into an AI-powered 3D imaging system, giving physicians real-time navigation and enhanced visualization to perform more precise bronchoscopic biopsies. The technology supports earlier and more accurate diagnoses of pulmonary nodules—helping improve patient outcomes while keeping procedural costs low.