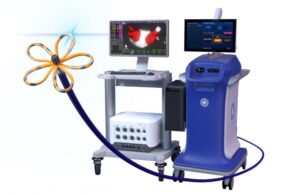
Farawave Nav and the Faraview software make up part of the company’s Farapulse pulsed field ablation (PFA) system. Boston Scientific initially won FDA approval for Farapulse in January 2024. In doing so, it became second company to earn an FDA nod for PFA that treats AFib. Medtronic picked up the first approval for PFA to treat paroxysmal and persistent AFib in December 2023.
Farawave Nav and Faraview combine to provide visualization for cardiac ablation procedures to treat AFib. They’re compatible exclusively with Boston Scientific’s existing cardiac mapping technology and its latest offering, the Opal HDx mapping system.
Boston Scientific won FDA approval for the catheter and software last October.