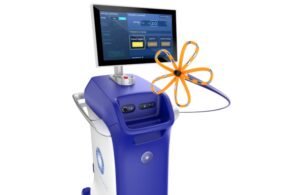
Farapulse, one of the leading PFA systems for treating AFib, received a much-anticipated FDA approval last year. The system treats AFib by delivering pulsed field energy through a catheter to ablate heart tissue.
Its updated labeling now includes approval for treating drug-refractory, symptomatic persistent atrial fibrillation (AFib). In these cases, the heart beats abnormally for at least seven days. The approval updates the instructions for use for both the Farawave PFA catheter and the Farawave Nav PFA catheter to include treatment for persistent AFib.
Boston Scientific shared findings from the ADVANTAGE AF trial in April that supported the expanded labeling. The company said at the time that it anticipated FDA approval for persistent AFib in the second half of 2025.