The certification, issued by Intertek Medical Notified Body AB (NB 2862) under Part II MDR 2002, Annex II, confirms that Bot Image’s Quality Management System and product design comply with all applicable UKCA safety and performance requirements.
Certificate No.: 85320228106
Effective Date: 31 October 2025
Expiry: 20 September 2029
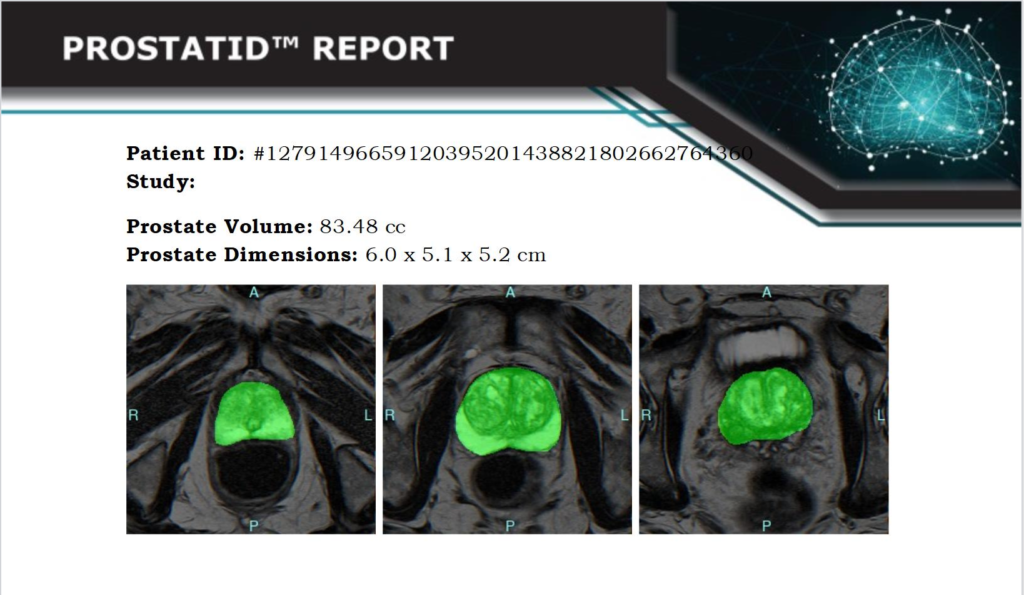
Scope: Image analysis software for the detection of prostate lesions
“This CE certification represents a pivotal step in our mission to make early, accurate prostate cancer detection accessible to clinicians and patients worldwide,” said Mike “Bing” Crosby, Chief Commercial Officer at Bot Image, Inc. “With FDA and EU clearance already in place, ProstatID® is now positioned to reach healthcare systems across the EU, UK and other countries accepting any of these certifications, where consistent, high-accuracy prostate MRI interpretation is urgently needed.”