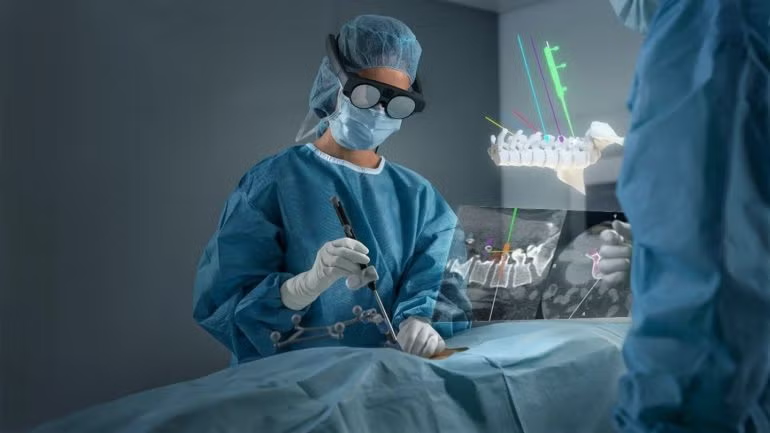
Digital medical technology company Brainlab has announced 510(k) clearance for its Spine Mixed Reality Navigation by the Food and Drug Administration, marking the system’s official launch in the US.
This surgical navigation tool provides mixed reality support, enhancing the “ergonomic view” for surgeons by integrating crucial navigation data directly into their line of sight during procedures.
The solution leverages the company’s optical navigation system technology and the latest advancements in mixed reality.