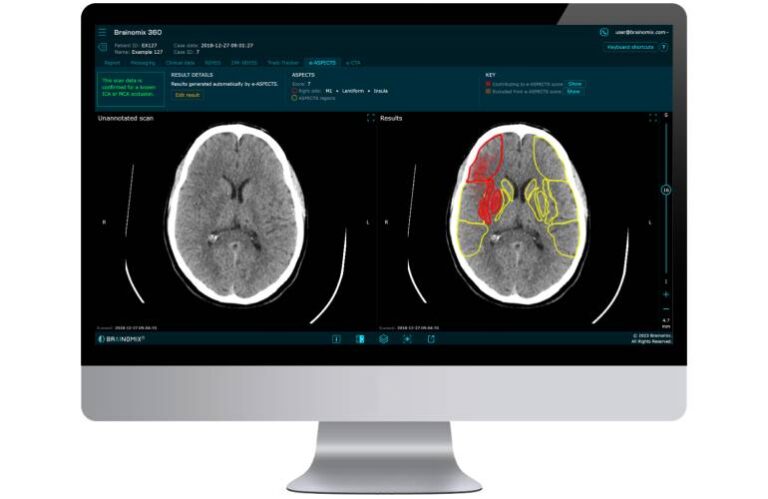
FDA clearance covers a novel, patented feature that enables physicians to assess ischemic core volume from universally available non-contrast CT (NCCT) images through the 360 Stroke platform. Studied and validated by leading U.S. stroke centers, the new feature demonstrated equivalency to CT perfusion and MRI-derived core volume assessments.
Powered by state-of-the-art AI algorithms, 360 Stroke provides real-time interpretations of brain scans. It aids in the treatment and transfer decisions for stroke patients, according to the company.
Brainomix says its latest feature addresses a longstanding unmet need in stroke triage. It enables physicians across stroke networks to access expert-level insights for decision-making using routinely available brain scans.