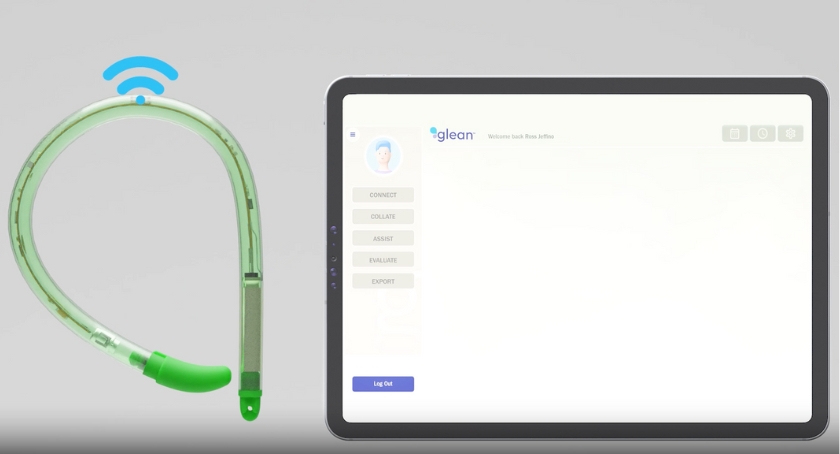
Glean, a comprehensive system, enables wireless, catheter-free urodynamics. Bright Uro aims to provide clinicians with more accurate data and actionable insights to aid in clinical decision-making. Glean features an insertion tool and a Bluetooth-enabled pressure sensor in a flexible silicone tube. It also has a software app for use by clinicians and patients and a uroflowmeter to sense volume and flow.
Irvine, California-based Bright Uro designed Glean to support patients with lower urinary tract dysfunction (LUTD) and other urological conditions. It moves beyond current catheter-based urethral pressure testing, which the company says is uncomfortable for patients and often produces imprecise data.
Glean, meanwhile, offers ease of use, delivers more accurate data and provides a more comfortable experience, Bright Uro says.