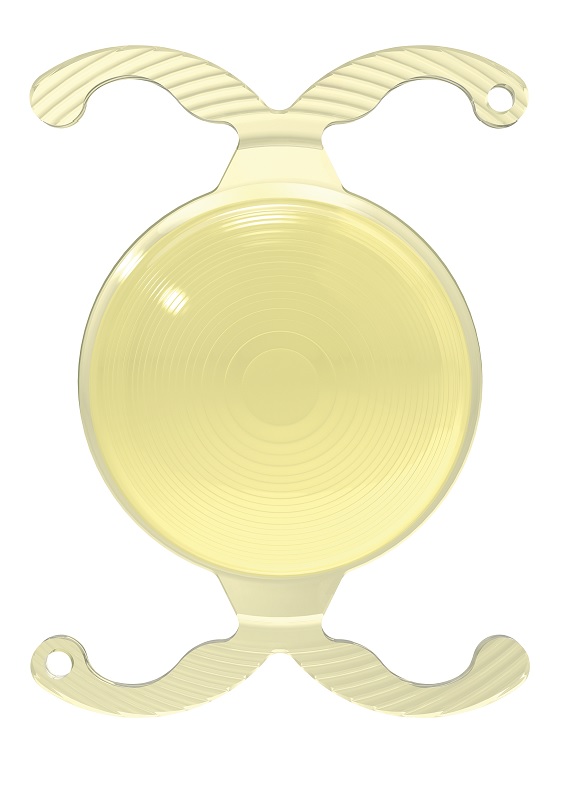
Waltham, Massachusetts-based BVI describes FineVision HP as the world’s first trifocal IOL, bringing in a new era of premium IOL offerings. FineVision HP features a patented diffractive optic design, delivery balanced vision at far, intermediate and near distances. The IOL won FDA approval for cataract surgery in October 2025.
BVI said the first clinical cases represent the transition from regulatory approval to real-world U.S. surgical practice. It expands the company’s strategy to expand its premium refractive portfolio in the world’s largest ophthalmic market.