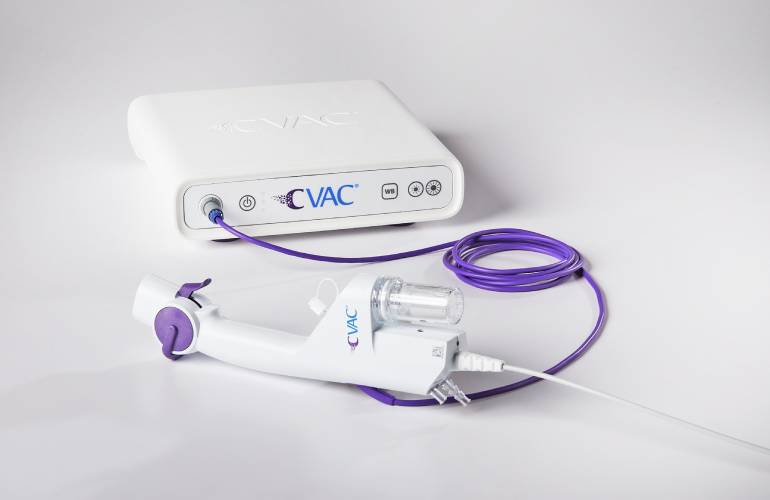
PLEASANTON, Calif.–(BUSINESS WIRE)– Calyxo, Inc., a medical device company developing innovative solutions for patients with kidney stones, announced that it has received FDA clearance for its new, redesigned CVAC System, which enables a minimally invasive approach to kidney stone treatment. More than 50 procedures have already been completed by 12 urologists with the new CVAC System, yielding strong patient outcomes and consistently positive physician feedback.
Clinical studies have shown that residual stone fragments are associated with a 20%-44% incidence of post-procedure problems, including pain, infection, emergency department visits, hospitalization and need for retreatment.
Steerable ureteroscopic renal evacuation (SURE) using the original CVAC Aspiration System has been used to successfully treat more than 1,500 patients in the U.S., demonstrating that vacuum aspiration of stone fragments improves clinical outcomes, with 97% volumetric stone clearance and a high likelihood of avoiding the need for a secondary or more invasive procedure (according to clinical study data collected in patients treated with the device).