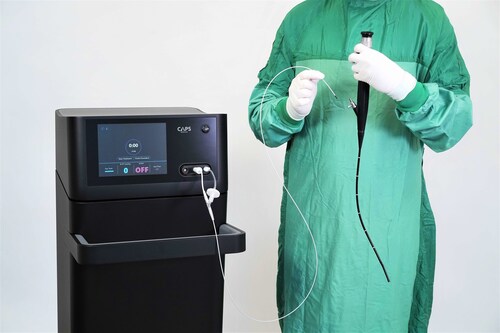
CAPS Medical has successfully completed its first-in-human clinical study, treating approximately 70 tumors and demonstrating durable complete response rates across short and long term follow up. The study showed an improved safety profile with no adverse events, no damage to surrounding healthy tissue, and excellent patient tolerance including procedures performed without general anesthesia. The company’s goal is to replace traditional transurethral resection of bladder tumors (TURBT), hospitalization, and general anesthesia with a simple, office-based procedure that allows patients to be treated earlier, with less risk, faster recovery, and lower cost.
Ilan Uchitel, CEO & Co-Founder of CAPS Medical, commented: “Receiving Breakthrough Device Designation is a strong validation of the clinical need for a safer, more accessible solution for NMIBC patients and of the differentiated potential of our PlasmaSure™ platform. This designation provides priority review and interactive communication with the FDA, more flexible clinical study design, and promising potential eligibility for CMS’s Transitional Coverage for Emerging Technologies pathway, supporting earlier and more predictable reimbursement.Our team is committed to turning this innovation into a new standard of care.”