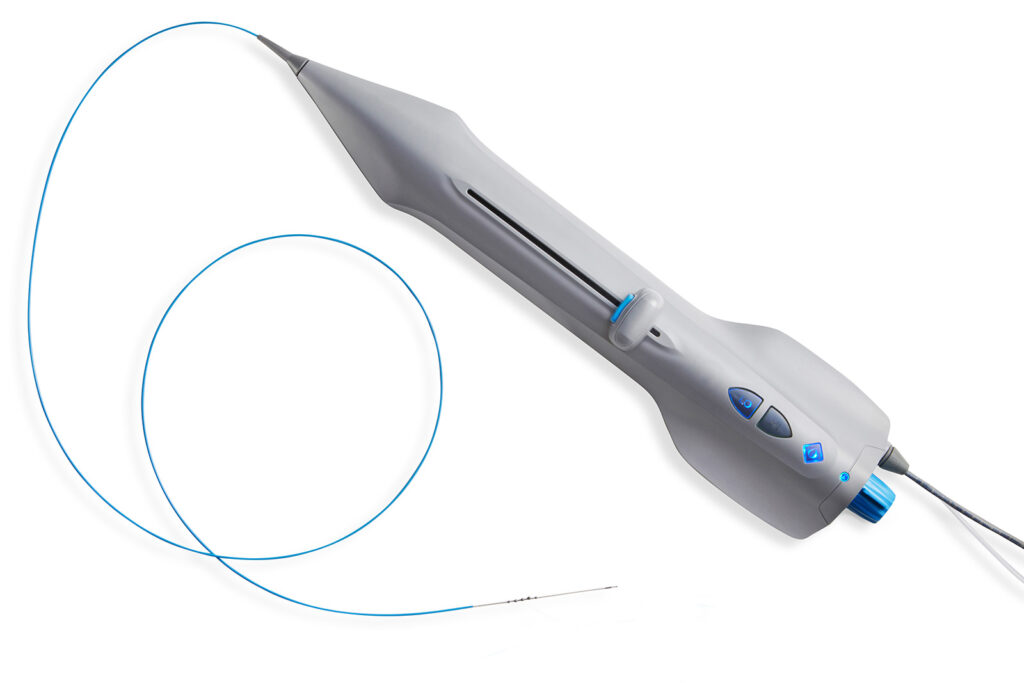
Cardio Flow announced the successful completion of its first commercial cases in leading medical centers across the U.S. The initial cases were performed by Drs. Craig Walker, Pradeep Nair, and McCall Walker of the Cardiovascular Institute of the South (CIS) at the Terrebonne General Health System in Houma, LA.
FreedomFlow treats plaque blockages in the legs, and its unique technology gives physicians greater versatility in treating multiple arteries and multiple blockages in the same vessel—all with a single device. The first patient treated at Terrebonne had complex arterial anatomy and multiple blockages with extensive calcium, including a chronic total occlusion. FreedomFlow’s dynamic new mechanism of action demonstrated its effectiveness in these initial procedures.
Dr. Pradeep Nair, M.D., an interventional cardiologist in Houma, LA, and a recognized leader in the treatment of PAD and critical limb-threatening ischemia commented: “Peripheral arterial blockages are extremely common, especially in our patients with heart disease. This new technology allows us to treat a wide range of blockages effectively from the ankle to the hip, with the goal of saving limbs—and ultimately our patients’ lives and the quality of those lives.”