Essentia Health launches new Alzheimer’s detection test
The test detects amyloid plaques, abnormal brain protein deposits linked to cognitive impairment and a key indicator of Alzheimer’s.

The test detects amyloid plaques, abnormal brain protein deposits linked to cognitive impairment and a key indicator of Alzheimer’s.
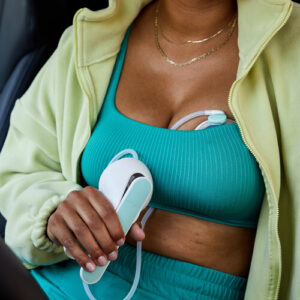
SAN FRANCISCO, Sept. 9, 2025 /PRNewswire/ — Willow Innovations, Inc. (Willow), the Femtech leader and inventor of the first fully in-bra wearable breast pump, is once again revolutionizing the pumping experience with the launch of its newest innovation.

The NHS announced a first-of-its-kind initiative in England, offering automated insulin delivery systems for pregnant women with diabetes.

OKLAHOMA CITY, Sept. 4, 2025 /PRNewswire/ — Biozen, LLC a digital health innovator integrating advanced biosensors with proprietary algorithms, will present its cuffless blood pressure technology at the American Heart Association (AHA) Hypertension Scientific Sessions on Sept. 5 at 9:00 a.m. in Baltimore, Maryland (Poster Session 2, Poster #FR443).
Ophthalmic device company BVI Medical unveiled its new Virtuoso phaco-vitrectomy surgical platform.

Daye has just launched a comprehensive at-home hormone testing service across the UK, and Med-Tech Insights spoke to Valentina Milanova, founder & CEO of Daye to find out more.

Tasso, a specialist in patient-centric blood collection, and SheMed, a UK-based women’s healthcare company, have teamed up to deliver safer, more personalised weight loss treatments through convenient at-home diagnostics.
The technology is compatible with all PTFE grafts and was studied extensively in the company’s pivotal clinical trial
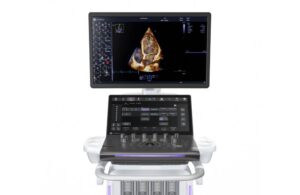
GE HealthCare (Nasdaq: GEHC)+
today announced the launch of Vivid Pioneer, its newest, most advanced cardiovascular ultrasound system.
A first-of-its-kind Blood Pressure Treatment Efficacy Calculator built on data from nearly 500 randomized clinical trials in over 100,000 people allows doctors to see how much different medications are likely to lower blood pressure.