
U-Pump takes next steps with AI-powered insulin patch pump
U-Pump announced on social media that it reached a new milestone in its development of an AI-powered, reusable insulin patch pump.

U-Pump announced on social media that it reached a new milestone in its development of an AI-powered, reusable insulin patch pump.
SCOTTSDALE, Ariz., March 20, 2025 /PRNewswire/ — Anuncia Medical, Inc. (“Anuncia”), a pioneering company in CSF management and neurocritical care, has received Breakthrough Device Designation from the FDA for its ReFlow® EVD, an innovative solution for external ventricular drains (EVDs) used to manage brain swelling and elevated intracranial pressure.

FRANKLIN LAKES, N.J., March 20, 2025 /PRNewswire/ — BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today announced the first patient treated in an Investigational Device Exemption (IDE) clinical trial intended to advance BD’s efforts to achieve Premarket Approval (PMA) from the U.S. Food and Drug Administration (FDA) for the use of GalaFLEX LITE™ Scaffold in decreasing capsular contracture (CC) recurrence during breast revision surgery.

Medtronic (NYSE:MDT) today announced real-world data supporting the use of its smart insulin pen and CGM sensor combination.

Tandem Diabetes Care (Nasdaq:TNDM) announced today that it launched its next-generation Control-IQ+ automated insulin delivery algorithm in the U.S.
Bausch + Lomb announced today that it launched Arise, its intelligent, cloud-based lens-fitting system, in the U.S.

Vivani Medical (Nasdaq:VANI) announced today that it successfully administered the first GLP-1 implant in its LIBERATE-1 clinical trial.

Think Surgical today announced the first successful use of Waldemar Link’s LinkSymphoKnee using the TMINI Miniature Robotic System.
Application covering a wide range of indications is in line with trends in de-escalation of surgery and growth in minimally invasive cryoablation procedures, pointing to strong potential for increasing demand
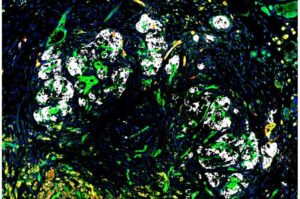
Adding engineered human blood vessel-forming cells to islet transplants boosted the survival of the insulin-producing cells and reversed diabetes in a preclinical study led by Weill Cornell Medicine investigators. The new approach, which requires further development and testing, could someday enable the much wider use of islet transplants to cure diabetes.