
Senseonics, Ascensia launch Eversense 365 year-long CGM in the U.S.
Ascensia Diabetes Care announced today that it launched the Eversense 365 CGM system from Senseonics (NYSE:SENS) in the U.S.

Ascensia Diabetes Care announced today that it launched the Eversense 365 CGM system from Senseonics (NYSE:SENS) in the U.S.
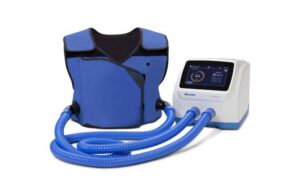
Baxter (NYSE: BAX)+
today unveiled its next-generation airway clearance system, The Vest advanced pulmonary experience (APX) system.

Johnson & Johnson MedTech‘s Shockwave Medical today announced the full U.S. launch of its Shockwave E8 peripheral IVL catheter.
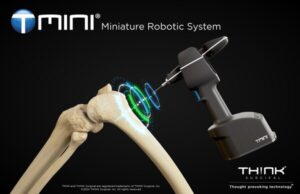
FREMONT, Calif., Sept. 10, 2024 /PRNewswire/ — THINK Surgical, Inc., an innovator in the field of orthopedic surgical robots, today announced the successful completion of the first total knee arthroplasty procedure utilizing its new TMINI® Miniature Robotic System version 1.1 following recent FDA 510(k) clearance.

FLOWOOD, Miss., Sept. 10, 2024 /PRNewswire/ — Zavation Medical Products, LLC (“Zavation”), an innovator in spinal device technology, proudly announces the launch of its latest product—the Varisync® ALIF System. Engineered to streamline ALIF procedures, the Varisync system offers customization and efficiency with two spacer fixation options: anchors and screws.

BETHLEHEM, Pa., Sept. 10, 2024 /PRNewswire/ — Tyber Medical LLC, a leading orthopedic device manufacturer specializing in private label implants for the extremity, trauma, and spine markets, is proud to announce that its PEEK ToeGrip Hammer Toe implant family has received Medical Device Regulation (MDR) certification from TÜV Rheinland. This prestigious certification marks the first time a hammer toe implant has achieved both FDA 510(k) clearance and MDR certification, setting a new standard for orthopedic implants.
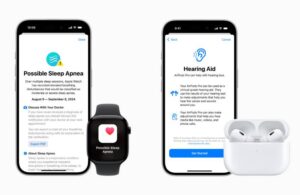
Apple today unveiled new sleep and hearing health features for its Apple Watch and AirPods Pro 2, expanding its health metrics offerings.

Abbott (NYSE: ABT)+
today announced a major product launch, broadening the population that can utilize its continuous glucose monitoring (CGM) technology.

Fresenius Medical Care announced today that it launched the newest version of its home hemodialysis (HHD) machine, the NxStage Versi HD with GuideMe software.

Women are offered the opportunity to benefit from Carevix at the Peterborough City Hospital – North West Anglia NHS Foundation Trust.