
Johnson & Johnson MedTech launches mobile simulator for breast procedures
Johnson & Johnson (NYSE: JNJ)+
announced that it launched the Arbrea Breast Simulator Surgeon App for Mentor in the U.S.

Johnson & Johnson (NYSE: JNJ)+
announced that it launched the Arbrea Breast Simulator Surgeon App for Mentor in the U.S.

Sequel Med Tech and Diabeloop today announced a collaboration to integrate a new algorithm into the twiist automated insulin pump.

Medtronic this week launched its OmniaSecure defibrillation lead in the U.S., describing it as the world’s smallest.

Peezy Midstream is available to Primary Care Providers, showing excellent clinical evidence around reduced false-positives and reduced specimen contamination evidenced by a Public Health Wales GP study, showing a 66% saving on lab spend.
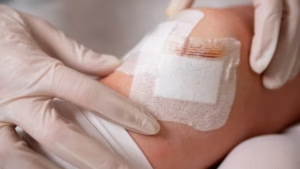
The B3 GEL System maintains biomechanical separation throughout the essential healing window.

The company will use the money to try to establish its cuffless device as a go-to option for 24-hour blood pressure monitoring in the U.S.

Picard Medical, the company behind the SynCardia Total Artificial Heart (TAH), announced today that it plans to launch a new, FDA-cleared artificial heart accessory.
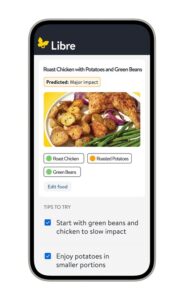
ABBOTT PARK, Ill., Jan. 5, 2026 /PRNewswire/ — Abbott (NYSE: ABT), a leading healthcare company, today unveiled Libre Assist,1 a groundbreaking feature within the Libre app5 designed to help the millions of people living with diabetes in the U.S. better understand how the foods they eat affect their glucose levels. 1,2 Unlike traditional food logging apps that only give feedback after a meal is logged, Libre Assist1 helps people make informed mealtime decisions before they eat. Abbott is launching the new technology during CES 2026 in Las Vegas.

LAS VEGAS, Jan. 3, 2026 /PRNewswire/ — Today at CES 2026, Sumbu announced its Exo-S3 line of dual-vector exoskeletons designed for the general public: the Exo-S3, Exo-S3 Pro and Exo-S3 Ultra. The series is the world’s first commercially available dual-vector exoskeleton designed to support human movement across real-world terrain. This release establishes a new market for lightweight, AI-powered wearable mobility products.

Theracor is a sheet device derived from human umbilical cord extracellular matrix (ECM) intended to cover, protect, and provide a moist wound environment