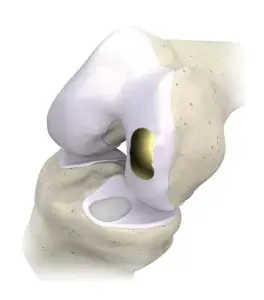
Overture Orthopaedics Announces Milestone $1.0Mn in Sales of its OvertureTi Knee Resurfacing System
OvertureTi Knee Resurfacing System implants are designed specifically as an alternative when it is still too early for arthroplasty

OvertureTi Knee Resurfacing System implants are designed specifically as an alternative when it is still too early for arthroplasty

In 2025, the medtech industry saw another year full of all kinds of deals in the mergers and acquisitions (M&A) arena.

As another year winds down, it’s safe to say that investment in medtech is not stopping any time soon.
New production capacity strengthens MPM Medical’s position as a leading U.S. manufacturer and follows the company’s recent launch of collagen at-home wound care kits
IRVINE, Calif., Dec. 16, 2025 /PRNewswire/ — OrthAlign, Inc., a global leader in surgical navigation technologies, today announced the expansion of its Lantern® Hip platform to include support for posterior-based approaches in total hip arthroplasty (THA).
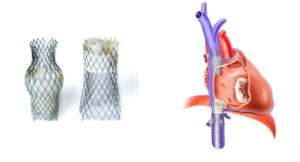
P&F USA, Inc., the U.S. subsidiary of heart valve manufacturer P&F Products and Features GmbH, today announced that the U.S. Food and Drug Administration (FDA) has approved initiation of the TRICAV II Pivotal Trial.

NES ZIONA, Israel, Dec. 8, 2025 /PRNewswire/ — EyeYon Medical Ltd., a global leader in ophthalmic innovation, today announced that the U.S. Food and Drug Administration (FDA) has granted Investigational Device Exemption (IDE) approval to initiate a U.S. clinical study of EndoArt®, the world’s first synthetic endothelial layer for the treatment of chronic corneal edema. EndoArt® is currently designated by the FDA as a Breakthrough Device.
SS Innovations (Nasdaq:SSII) announced today that it submitted a 510(k) premarket notification to the FDA for its Mantra surgical robot.

Commercialisation efforts for mjn-neuro’s EPISERA sensor in Europe will be handled by Neuraxpharma.

Microbot is collaborating with Emory to establish an Endovascular Robotics Program in interventional radiology to enhance the growing and evolving specialty field.