VSI® Performs World’s First Robotic Minimally Invasive Bertolotti Resection Surgery
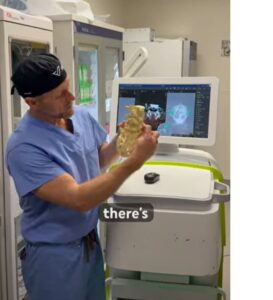
RESTON, Va., Nov. 25, 2025 /PRNewswire/ — VSI® Spine Surgeon Dr. Christopher Good has achieved a historic milestone in robotic spine surgery, performing the world’s first minimally invasive robotic Bertolotti’s resection surgery at Reston Hospital Center (HCA Virginia Health System). This first-ever procedure introduces a transformative surgical option for patients suffering from chronic low back pain caused by Bertolotti syndrome, an underdiagnosed spinal condition affecting an estimated 4-8% of the population.