
Relief Cardiovascular announces first human use of smart heart implant
Relief Cardiovascular today announced the successful first-in-human use of its transcatheter smart heart implant.

Relief Cardiovascular today announced the successful first-in-human use of its transcatheter smart heart implant.

MADISON, Wis., Sept. 26, 2025 /PRNewswire/ — Accuray Incorporated (NASDAQ: ARAY) announced today that the company is advancing its legacy of leadership in adaptive radiotherapy with the introduction of the Accuray Stellar™* Solution. Initially for the U.S. market, the new Accuray technology is not just a system, it’s a solution, comprised of a comprehensive tool set designed to empower clinical teams to address the evolving treatment needs of patients undergoing radiotherapy.
The newly issued patent strengthens Nexalin’s expanding intellectual property estate, supporting the Company’s DIFS platform and ongoing clinical and regulatory initiatives
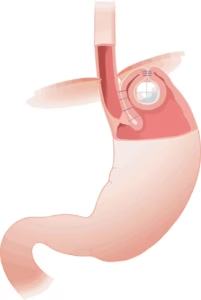
VADUZ, Liechtenstein, Sept. 25, 2025 /PRNewswire/ — Implantica AG (publ.), a medtech company at the forefront of introducing advanced technology into the body, including a unique device RefluxStop® for the treatment of acid reflux
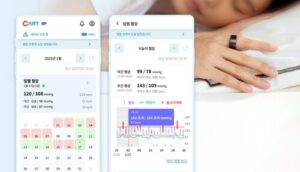
SEOUL, South Korea, Sept. 25, 2025 /PRNewswire/ — Sky Labs announced the official launch of CART BP, a ring-type personal blood pressure monitor designed for everyday use.

STAMFORD, Conn., Sept. 25, 2025 /PRNewswire/ — Today Apiject Systems, Corp. (Apiject, www.apiject.com) announced its submission for regulatory approval of its New Drug Application (NDA) to the Food and Drug Administration (FDA) for the world’s first injectable medicine to use Apiject’s single dose, single use prefilled plastic syringe.
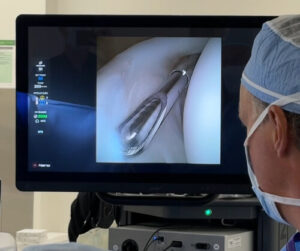
NAPLES, Fla., Sept. 23, 2025 /PRNewswire/ — Arthrex, a global leader in minimally invasive surgical technology, announced the launch of NanoNeedle™ scope 2.0, the latest version of the company’s minimally invasive imaging platform. The technology was recently used for the first time in a clinical case by Kyle Anderson, MD, at Anderson Sports Medicine in Bingham Farms, Michigan.
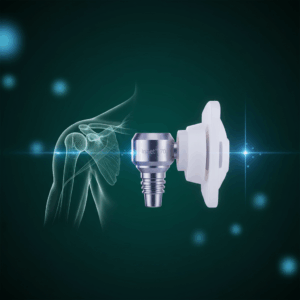
GRAND RAPIDS, Mich., Sept. 22, 2025 /PRNewswire/ — Shoulder Innovations, Inc. (Shoulder Innovations, or the company) (NYSE: SI), a commercial-stage medical technology company exclusively focused on transforming the shoulder surgical care market, today announced the full commercial launch of the InSet™ 70 Humeral Stem (“InSet™ 70”).
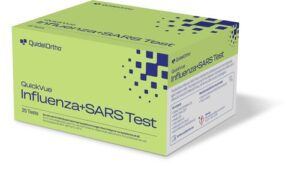
SAN DIEGO, Sept. 22, 2025 /PRNewswire/ — Building on its leadership in point-of-care diagnostic testing, QuidelOrtho Corporation (Nasdaq: QDEL) (“QuidelOrtho”), a leading global provider of innovative in vitro diagnostic technologies, is expanding its QUICKVUE portfolio with the QUICKVUE Influenza + SARS Test, a CLIA-waived, 510(k)–cleared rapid, easy-to-use immunoassay designed for professional use in physician office laboratories, urgent care centers, emergency departments, pharmacies and decentralized hospital labs.

J&J’s Shockwave Javelin intravascular lithotripsy catheter treats people with peripheral artery disease.