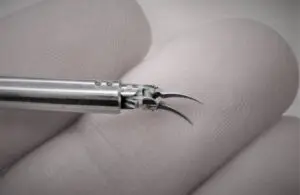
Medical Microinstruments wins FDA nod for surgical robot instruments
Medical Microinstruments (MMI) announced today that the FDA granted 510(k) clearance for its NanoWrist surgical robot instruments.

Medical Microinstruments (MMI) announced today that the FDA granted 510(k) clearance for its NanoWrist surgical robot instruments.

The approval is said to cover a range of clinical indications and supports use in both acute and chronic patients.

Resmed (NYSE: RMD)+
announced today that it received FDA clearance for its Smart Comfort personalized therapy offering.
Healthcare professionals can access the 1Bio AI-Acute toolbox via the company’s 1Bio platform.

Medtronic (NYSE: MDT)+
announced that the FDA approved expanded labeling for its deep brain stimulation (DBS) offering.

Cardiawave announced that it received CE mark approval for Valvosoft, its non-invasive therapeutic alternative for treating aortic stenosis (AS).
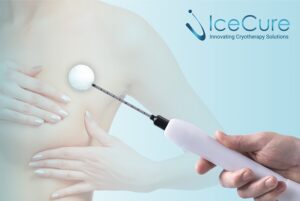
CAESAREA, Israel, Dec. 5, 2025 /PRNewswire/ — IceCure Medical Ltd. (NASDAQ: ICCM) (“IceCure”, “IceCure Medical” or the “Company”), developer of minimally-invasive cryoablation technology that destroys tumors by freezing as an option to surgical tumor removal, today announced it received a Notice of Allowance for a patent from the China National Intellectual Property Administration for its invention titled “Cryogen Flow Control” which relates to its next-generation XSense™ cryoablation system and probes.

Insulet (Nasdaq: PODD)+
announced today that it received FDA 510(k) clearance for new enhancements to its Omnipod 5 system.
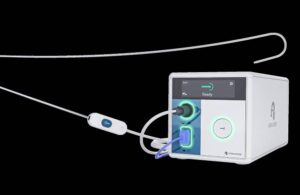
Atraverse Medical announced today that it received FDA clearance for its fully integrated Hotwire transseptal access system.

Boston Scientific (NYSE: BSX)+
announced recently that it received CE mark for its Farapoint pulsed field ablation (PFA) catheter.