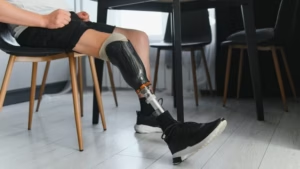
FDA grants IDE approval for Renerva’s trial of nerve capping device
The trial will enroll patients who are undergoing nerve management procedures for severe neuropathic pain.

The trial will enroll patients who are undergoing nerve management procedures for severe neuropathic pain.

MED-EL’s cochlear implants are now FDA-approved in children seven months and older with bilateral sensorineural hearing loss (SNHL).

The approval is a major endorsement of Biomoneta’s scientific breakthrough and an important inflection point for Beyond Next Ventures India (BNV India)

Avance is an acellular nerve scaffold for the treatment of adult and pediatric patients aged 1 month or older with sensory, mixed, and motor peripheral nerve discontinuities
SURREY, BC, Dec. 4, 2025 /PRNewswire/ – mlHealth 360 today announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance (K250694) for Scaida BrainCT-ICH, an AI-powered triage solution designed to address critical bottlenecks in acute care radiology.

MIDDLETON, Wis., Dec. 3, 2025 /PRNewswire/ — Natus Medical Incorporated announced the electrographic status epilepticus diagnosis capability of its BrainWatch point-of-care EEG solution, featuring integrated Persyst analysis software which has received 510(k) clearance from the U.S. Food and Drug Administration.

BEIJING, Dec. 3, 2025 /PRNewswire/ — Wingderm® announces that its Renuva 1550nm non-ablative fractional laser system has obtained MDR certification in the European Union, marking a significant regulatory milestone for the device within the EU market.
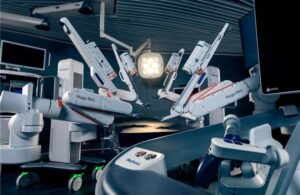
Medtronic (NYSE: MDT)+
announced today that the FDA cleared its Hugo robotic-assisted surgery system for use in urologic surgical procedures.
The test, which runs on the cobas liat system, is said to deliver results in 15 minutes.
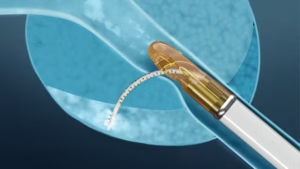
The Vanquish device’s clinical data demonstrated an elimination of MRI visible intermediate risk disease in 91% of patients after a single procedure.