
FDA clears Augmedics’ AR headset for use with spine surgery system
The company will introduce the headset at the North American Spine Society Annual Meeting in the US on 14 November.

The company will introduce the headset at the North American Spine Society Annual Meeting in the US on 14 November.
ViTAA’s FDA clearance on its AiORTA tool represents the first part of its planned full-suite aortic care platform.

ALTSTÄTTEN, Switzerland and BOSTON, Nov. 13, 2025 /PRNewswire/ — icotec, the pioneer of implantable devices made from BlackArmor® Engineered Carbon/PEEK, today announced that the U.S. Food and Drug Administration (FDA) has cleared the CMORE® CT System for use in the cervicothoracic spine.

BREA, Calif., Nov. 13, 2025 /PRNewswire/ — Rapid Nexus Nanotech Wound Solutions, Inc., a California-based med-tech company focused on advanced wound care, today announced it has received FDA 510(k) clearance for its Hemastyl gel device—marking a historic milestone as the first company to directly target the underlying reason chronic wounds fail to heal.
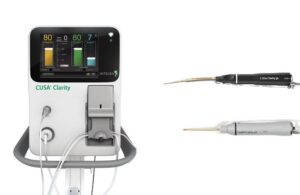
Integra LifeSciences (Nasdaq: IART)+
announced that it received FDA 510(k) clearance for its CUSA aspirator system for cardiac surgeries.

LISLE, Ill., Nov. 12, 2025 /PRNewswire/ — Hubly Surgical today announced FDA 510(k) clearance expanding Hubly Auto-Stop Drill indications to include spinal decompression procedures. To date, many thousands of neurosurgical patient lives have been saved using Hubly Auto-Stop Drills; the device’s SMART auto-stop halts rotation and prevents forward plunge at the instant of skull penetration, preventing over-drilling into patients’ brains. The new clearance expands use to laminectomy and laminotomy for spinal decompression to drill through the vertebral lamina and protect patients from damage to the spinal cord.
Zap Surgical’s ZAP Axon system aims to make radiosurgery planning more straightforward and efficient.

Go-Pen announced today on social media that it received CE mark approval for its user-fillable insulin pen.
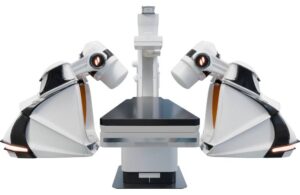
Stereotaxis (NYSE:STXS) announced today that it received FDA 510(k) clearance for its next-generation GenesisX surgical robot.
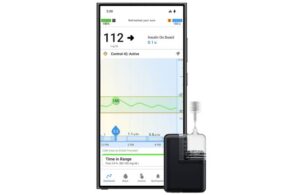
Tandem Diabetes Care (Nasdaq:TNDM) announced today that it received FDA approval for the Android version of its Mobi mobile app