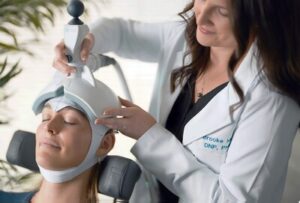
Ampa Launches Nationwide Rollout of Portable FDA-Cleared Brain Stimulation System Following $8.5M Oversubscribed Funding Round
PALO ALTO, Calif., Oct. 22, 2025 /PRNewswire/ — Four months after emerging from stealth with FDA clearance of the Ampa One system and an oversubscribed $8.5 million round led by Nexus NeuroTech Ventures, Ampa today announced its nationwide rollout. This milestone marks a major step in bringing advanced, non-invasive brain stimulation to patients across the country and brings Ampa’s total funding to $18 million.