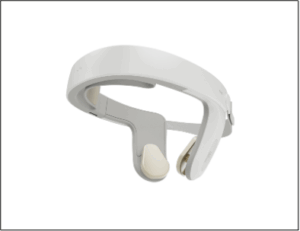
Nexalin Technology Announces Issuance of U.S. Patent Covering HALO Clarity Device Featuring DIFS Technology
The newly issued patent strengthens Nexalin’s expanding intellectual property estate, supporting the Company’s DIFS platform and ongoing clinical and regulatory initiatives