
Centinel Spine’s prodisc C Vivo and C Nova systems obtain MDR certification
The company has completed registration and certification for its total disc replacement products, which are now CE-marked.

The company has completed registration and certification for its total disc replacement products, which are now CE-marked.

Johnson & Johnson (NYSE:JNJ) announced today that the FDA approved Inlexzo, its gemcitabine intravesical drug delivery system.
Neurescue announced today that it received CE mark approval for its device designed to treat non-shockable cardiac arrest.
Varian announced its Embozene microspheres have received CE mark for genicular artery embolisation (GAE) to treat knee osteoarthritis.

The FDA’s clearance of LIBERTY follows the completion of Microbot’s pivotal trial in April 2025, achieving a 100% success rate in robotic navigation.
ARC‑EX is the first system to receive a CE Mark in Europe specifically for improving hand and arm strength and sensation in adults with chronic, incomplete spinal cord injury.

Pulse Biosciences (Nasdaq:PLSE) announced today that the FDA granted investigational device exemption (IDE) for its nanosecond pulsed field ablation (nsPFA) system.

Lifeward (Nasdaq:LFWD) announced today that it received CE mark approval for its ReWalk 7 personal exoskeleton.

The company raised $250 million in July to expand its manufacturing capabilities and establish multiple teams to support the launch.
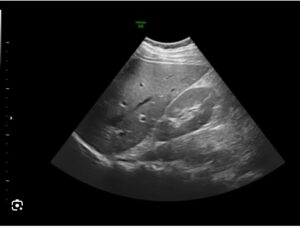
DENVER, Sept. 4, 2025 /PRNewswire/ — Ultrasound AI™, Inc., a pioneer in artificial intelligence for medical imaging, today announced that the United States Patent and Trademark Office has issued U.S. Patent No. 12,369,883, “Artificial Intelligence System for Determining Clinical Values through Medical Imaging.” The patent protects the company’s proprietary system for determining current or future clinical or laboratory values directly from non-invasive medical images such as ultrasound.