
Moon Surgical gets FDA nod for AI-enhancement for surgical robot
Moon Surgical announced today that it received FDA clearance for ScoPilot, a Nvidia-enabled platform for its Maestro surgical robot.

Moon Surgical announced today that it received FDA clearance for ScoPilot, a Nvidia-enabled platform for its Maestro surgical robot.

Lifeward (Nasdaq:LFWD) announced today that it received FDA 510(k) clearance for its latest-generation personal exoskeleton device, ReWalk 7.

Spineart and eCential Robotics today announced the receipt of FDA 510(k) clearance for the use of an application for robotic navigation.
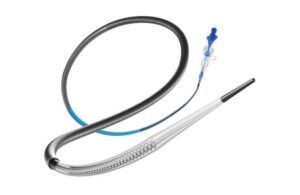
Perfuze announced today that it received FDA 510(k) clearance for its Zipline access catheter and secured significant funding.
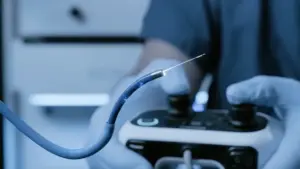
Using Nvidia and GE Healthcare technology, the robotic surgery system is intended to help doctors detect lung cancer earlier.
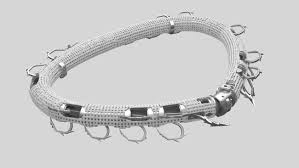
HERZLIYA, Israel and WILMINGTON, Del., March 13, 2025 /PRNewswire/ — Valcare Medical Inc., a leading innovator in transcatheter-based mitral solutions, today announced the U.S. Food and Drug Administration (FDA) has approved the AMEND™ Trans-Septal System for investigational device exemption (IDE) application to commence an Early Feasibility Study (EFS).

Johnson & Johnson MedTech (NYSE: JNJ)+
announced today that the FDA cleared its Monarch Quest technology for robotic-assisted bronchoscopy.

SALT LAKE CITY, March 12, 2025 /PRNewswire/ — For the thousands of patients in the United States requiring treatment for bone loss or defects caused by trauma or infections, Elute, Inc., a clinical stage company and emerging leader with a groundbreaking controlled and extended drug delivery platform, proudly announces U.S. Food and Drug Administration (FDA) approval of BonVie+™, a novel bone void filler implant.

Sooma Medical announced today that it received FDA investigational device exemption (IDE) for its transcranial direct current stimulation (tDCS) device.
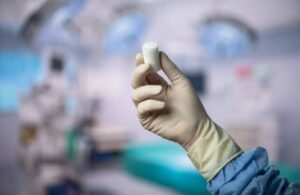
Miach Orthopaedics announced today that the FDA cleared an expanded indication for the company’s Bear implant for ACL tears.