
FDA approves Endotronix Cordella PA sensor system for heart failure treatment
Endotronix today announced it received FDA premarket approval for its Cordella PA Sensor System.

Endotronix today announced it received FDA premarket approval for its Cordella PA Sensor System.
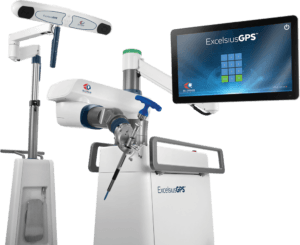
Globus Medical has won FDA clearance for its new ExcelsiusFlex orthopedics robot stereotaxic surgery in knee procedures and an expanded indication for its ExcelsiusHub system.

The designation recognises the novelty of the technology and the potential to provide a more effective treatment option for patients with coronary artery disease.
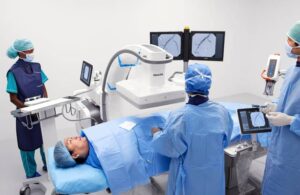
Philips (NYSE:PHG) announced today that it launched its now FDA-cleared Zenition 90 motorized mobile C-arm system.

aXess is a restorative arteriovenous dialysis conduit which allows vascular access for hemodialysis through the creation of a new, permanent, living vessel

The potential benefits of utilizing the CORUS™ Navigation Access System to perform a navigated CORUS fusion include:
> Enhanced accuracy and precision with surgical instruments and spinal implants, e.g., CAVUX FFS-LX.
> Improved visualization of the facet joints and other anatomical structures during procedures.
> Reduced risk of complications and shorter operative time.

With the FDA 510(k) clearance of the COR-12 wireless ECG device now integrated with the SpiroSphere®, sponsors can conduct comprehensive respiratory trials with cardiac safety ECG collection on one device.
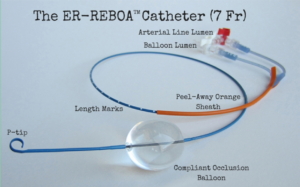
Temporary vessel occlusion a growing practice for trauma patients.
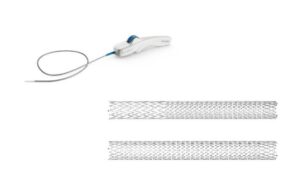
Philips (NYSE: PHG) today announced the first implant of its Duo venous stent system following FDA premarket approval.

Getinge announced today that it received FDA 510(k) clearance for its Talis +ACG (advanced clinical guidance) support software.