Novel biomarker predicts chemotherapy response in triple-negative breast cancer
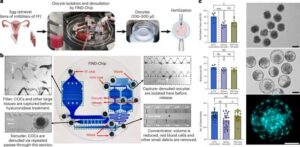
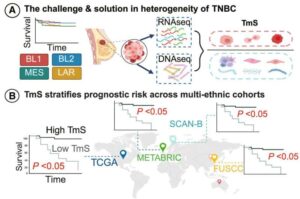
Researchers at The University of Texas MD Anderson Cancer Center have developed a new computational approach designed to better account for changes in gene expression within tumors relative to their unique microenvironments. This approach outperformed current methods for predicting chemotherapy response in patients with triple-negative breast cancer (TNBC).