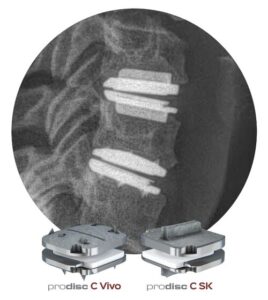
Centinel Spine® Receives Two-Level FDA Approval for prodisc® C Vivo and prodisc® C SK Match-the-Disc™ Cervical Total Disc Replacement Devices
WEST CHESTER, Pa., Oct. 14, 2025 /PRNewswire/ — Centinel Spine®, LLC (“the Company”), the leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), today announced U.S. Food and Drug Administration (FDA) Premarket Approval (PMA) for 2-level indications for the prodiscC Vivo and prodisc C SK Cervical TDR devices. Since receiving FDA approval for 1-level indications in July 2022, nearly 20,000 prodiscC Vivo and prodisc C SK spinal levels have been implanted in the U.S. by over 1,100 surgeons.