
FDA clears revision knee implant from Zimmer Biomet
Zimmer Biomet (NYSE: ZBH) announced today that it received FDA 510(k) clearance for its Persona Revision SoluTion Femur.

Zimmer Biomet (NYSE: ZBH) announced today that it received FDA 510(k) clearance for its Persona Revision SoluTion Femur.

MINNEAPOLIS and MISGAV, Israel, Feb. 27, 2025 /PRNewswire/ — Arcuro Medical Ltd., (“Arcuro”) today announced that it received FDA 510(k) clearance for its new SuperBall-RC™ system for use in rotator cuff repair procedures.

PALM COAST, Fla., Feb. 25, 2025 /PRNewswire/ — OrthoNovis, Inc is proud to announce that it has received U.S. Food and Drug Administration (FDA) clearance to market its innovative BPS Wrist Fracture System. www.orthonovis.com

TAMPERE, Finland, Feb. 14, 2025 /PRNewswire/ — Bioretec Ltd., a pioneer in absorbable orthopedic implants, has achieved a pivotal milestone with the successful completion of its CE mark approval process for the RemeOs™ Trauma Screw product portfolio
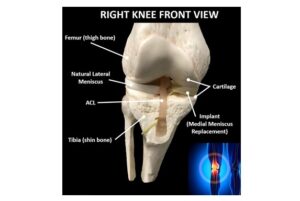
RICHMOND, Va. and ATLANTA, Jan. 29, 2025 /PRNewswire/ — OrthoPreserve, a company developing orthopedic implant solutions, announced today it has been granted both a Breakthrough Device Designation and Total Product Life Cycle Advisory Program (TAP) enrollment from the U.S. Food & Drug Administration (FDA) for Defender, a meniscus replacement implant.

Smith+Nephew (NYSE: SNN)+
announced that it received FDA clearance for its stemless anatomic total shoulder for the Aetos system.

The Osseofit devices are intended to match patients’ shoulder bone anatomy and preserve healthy bone in total shoulder replacement procedures.

WARSAW, Ind., Dec. 4, 2024 /PRNewswire/ — Zimmer Biomet Holdings, Inc. (NYSE and SIX: ZBH), a global medical technology leader, today announced U.S. Food and Drug Administration (FDA) 510(k) clearance of Persona® SoluTion™ Porous Plasma Spray (PPS®) Femur, a total knee implant component offering an alternative for patients with sensitivities to bone cement and/or metal.
The device has sold well in Europe, where the company said it has a 60% market share, but will be the first product of its type available in the U.S.
Corin announced that it received FDA 510(k) clearance for its Icona femoral stem implant for total hip arthroplasty (THA).