
FDA clears Exactech porous 3D tibial implant for knee replacement
Exactech announced today that it received FDA 510(k) clearance for its Truliant porous tibial tray, a 3D tibial knee implant.

Exactech announced today that it received FDA 510(k) clearance for its Truliant porous tibial tray, a 3D tibial knee implant.

CytexOrtho today said it has approval from the FDA to start a Phase 1 clinical trial evaluating the safety and efficacy of its absorbable hip implant in human patients.

Si-Bone today announced the first patient procedures with its FDA breakthrough device, the iFuse Torq TNT implant system.

CHICAGO, Oct. 3, 2024 /PRNewswire/ — Amphix Bio, a company developing a new class of regenerative medicine therapies, announced today it has received a Breakthrough Device designation from the U.S. Food and Drug Administration (FDA) for a drug-device combination product for bone regeneration. The designation covers the use of the therapeutic device to treat degenerative disc disease with transforaminal lumbar interbody fusion (TLIF) procedures.
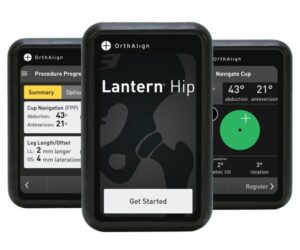
IRVINE, Calif., Oct. 3, 2024 /PRNewswire/ — OrthAlign, Inc., a privately held medical device company, announces FDA 510(k) clearance of their Lantern Hip handheld technology for direct anterior total hip arthroplasty with the patient in the supine position. Lantern Hip is the latest addition to the Lantern platform, joining existing applications for total knee, revision knee, and partial knee arthroplasty.

BETHLEHEM, Pa., Sept. 10, 2024 /PRNewswire/ — Tyber Medical LLC, a leading orthopedic device manufacturer specializing in private label implants for the extremity, trauma, and spine markets, is proud to announce that its PEEK ToeGrip Hammer Toe implant family has received Medical Device Regulation (MDR) certification from TÜV Rheinland. This prestigious certification marks the first time a hammer toe implant has achieved both FDA 510(k) clearance and MDR certification, setting a new standard for orthopedic implants.
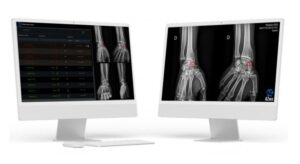
PARIS, Sept. 3, 2024 /PRNewswire/ — European MedTech startup AZmed, recognized as a leading company in AI for medical imaging, today announced that its Rayvolve solution has received 510(k) clearance from the Food and Drug Administration (FDA) for detecting fractures on pediatric X-rays. This milestone comes two years after receiving FDA clearance for adult fracture detection, positioning Rayvolve as a top-performing solution for detecting fractures in adult and pediatric patients. The clearance was supported by an independent study conducted with SimonMed Imaging, one of the largest outpatient imaging providers in the U.S.
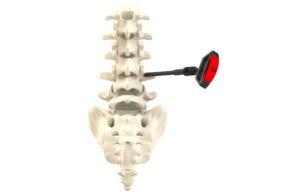
SurGenTec announced today that it received FDA 510(k) clearance for its proprietary B-MAN bone marrow aspirate kit.

Si-Bone this week announced it received FDA 510(k) clearance and FDA breakthrough device designation for its iFuse Torq TNT Implant System.

Enovis (NYSE: ENOV)+ announced today that it unveiled its STAR Ankle system with new e+ Polyethylene.